Label: ADENOSINE injection
- NDC Code(s): 71872-7335-1
- Packager: Medical Purchasing Solutions, LLC
- This is a repackaged label.
- Source NDC Code(s): 25021-318
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated December 16, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONSAGENT - ® Rx only
-
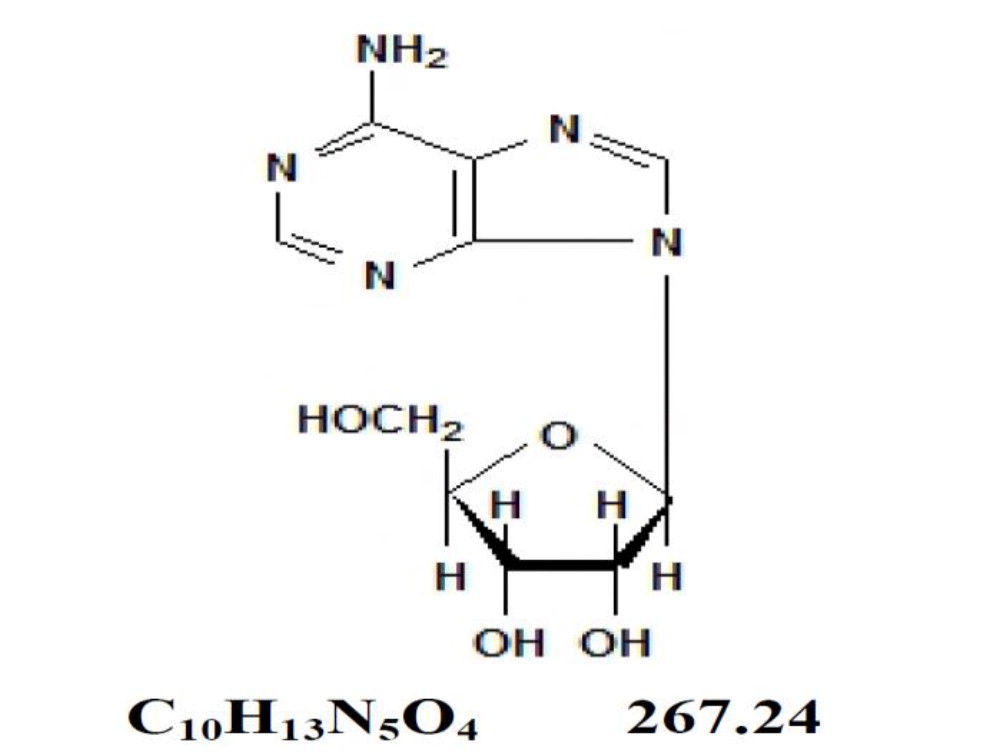
DescriptionAdenosine is an endogenous nucleoside occurring in all cells of the body. It is chemically 6-amino-9-β-D-ribofuranosyl-9-H-purine and has the following structural formula: Adenosine is a white ...
-
Clinical PharmacologyMechanism of Action - Adenosine Injection slows conduction time through the A-V node, can interrupt the reentry pathways through the A-V node, and can restore normal sinus rhythm in patients with ...
-
Indications and UsageIntravenous adenosine injection is indicated for the following. Conversion to sinus rhythm of paroxysmal supraventricular tachycardia (PSVT), including that associated with accessory bypass tracts ...
-
Contraindications(What is this?)Intravenous adenosine injection is contraindicated in: Second- or third-degree A-V block (except in patients with a functioning artificial pacemaker). Sinus node disease, such as sick sinus ...
-
WarningsHeart Block - Adenosine Injection exerts its effect by decreasing conduction through the A-V node and may produce a short lasting first-, second- or third-degree heart block. Appropriate therapy ...
-
PrecautionsDrug Interactions - Intravenous adenosine injection has been effectively administered in the presence of other cardioactive drugs, such as quinidine, beta-adrenergic blocking agents, calcium ...
-
Adverse ReactionsThe following reactions were reported with intravenous adenosine injection used in controlled U.S. clinical trials. The placebo group had a less than 1% rate of all of these ...
-
OverdosageThe half-life of adenosine injection is less than 10 seconds. Thus, adverse effects are generally rapidly self-limiting. Treatment of any prolonged adverse effects should be individualized and be ...
-
Dosage and AdministrationFor rapid bolus intravenous use only. Adenosine Injection, USP should be given as a rapid bolus by the peripheral intravenous route. To be certain the solution reaches the systemic circulation ...
-
How SuppliedAdenosine Injection, USP is supplied as a sterile non-pyrogenic solution in normal saline. NDCAdenosine Injection, USP (3 mg per mL)Package Factor - 25021-318-026 mg per 2 mL Single-Dose ...
-
REFERENCEPaul T, Pfammatter. J-P. Adenosine: an effective and safe antiarrhythmic drug in pediatrics. Pediatric Cardiology1997; 18:118-126 - SAGENT - ® Mfd. for SAGENT Pharmaceuticals ...
-
PRINCIPAL DISPLAY PANEL – VIAL LABEL(What is this?)Rx only - Adenosine Injection, USP - 6 mg per 2 mL - (3 mg per mL) For Rapid Bolus Intravenous Use
-
PRINCIPAL DISPLAY PANEL - OUTER PACKAGE NDC 71872-7335-1 - Adenosine Injection, USP - 6 mg/2 mL (3 mg/mL) 1 x 2 mL Single-Dose Vial - RX Only
-
INGREDIENTS AND APPEARANCEProduct Information