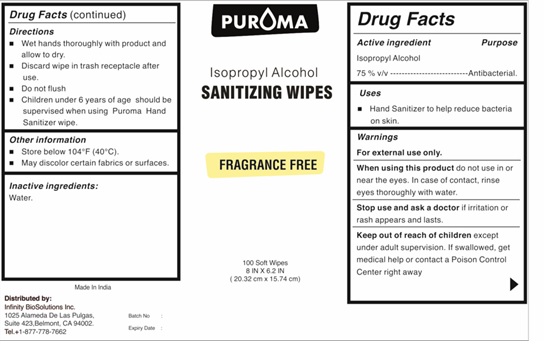
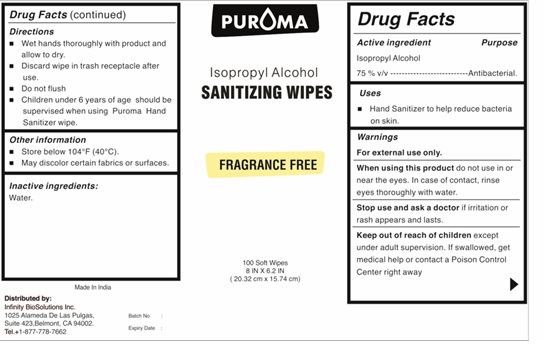
Label: PUROMA IPA HAND SANITIZER WIPE- isopropyl alcohol cloth
-
NDC Code(s):
80948-019-01,
80948-019-02,
80948-019-03,
80948-019-04, view more80948-019-05, 80948-019-06, 80948-019-07, 80948-019-08, 80948-019-09, 80948-019-10, 80948-019-11, 80948-019-12, 80948-019-13, 80948-019-14, 80948-019-15, 80948-019-16, 80948-019-17, 80948-019-18
- Packager: ZENITH MICRO CONTROL
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Product label
-
INGREDIENTS AND APPEARANCE
PUROMA IPA HAND SANITIZER WIPE
isopropyl alcohol clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80948-019 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 75 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80948-019-01 10 in 1 PACKAGE 04/27/2021 04/27/2021 1 66 mL in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC:80948-019-02 20 in 1 PACKAGE 04/27/2021 04/27/2021 2 132 mL in 1 PACKAGE; Type 0: Not a Combination Product 3 NDC:80948-019-03 40 in 1 PACKAGE 04/27/2021 04/27/2021 3 264 mL in 1 PACKAGE; Type 0: Not a Combination Product 4 NDC:80948-019-04 80 in 1 PACKAGE 04/27/2021 04/27/2021 4 528 mL in 1 PACKAGE; Type 0: Not a Combination Product 5 NDC:80948-019-05 120 in 1 PACKAGE 04/27/2021 04/27/2021 5 792 mL in 1 PACKAGE; Type 0: Not a Combination Product 6 NDC:80948-019-06 50 in 1 CANISTER 04/27/2021 04/27/2021 6 350 mL in 1 CANISTER; Type 0: Not a Combination Product 7 NDC:80948-019-07 80 in 1 CANISTER 04/27/2021 04/27/2021 7 560 mL in 1 PACKAGE; Type 0: Not a Combination Product 8 NDC:80948-019-08 100 in 1 CANISTER 04/27/2021 04/27/2021 8 700 mL in 1 CANISTER; Type 0: Not a Combination Product 9 NDC:80948-019-09 120 in 1 CANISTER 04/27/2021 04/27/2021 9 840 mL in 1 CANISTER; Type 0: Not a Combination Product 10 NDC:80948-019-10 10 in 1 POUCH 10/19/2021 10 66 mL in 1 POUCH; Type 0: Not a Combination Product 11 NDC:80948-019-11 20 in 1 POUCH 10/19/2021 11 132 mL in 1 POUCH; Type 0: Not a Combination Product 12 NDC:80948-019-12 40 in 1 POUCH 10/19/2021 12 264 mL in 1 POUCH; Type 0: Not a Combination Product 13 NDC:80948-019-13 80 in 1 CANISTER 10/19/2021 13 528 mL in 1 CANISTER; Type 0: Not a Combination Product 14 NDC:80948-019-14 120 in 1 CANISTER 10/19/2021 14 792 mL in 1 CANISTER; Type 0: Not a Combination Product 15 NDC:80948-019-15 50 in 1 CANISTER 10/19/2021 15 330 mL in 1 CANISTER; Type 0: Not a Combination Product 16 NDC:80948-019-16 100 in 1 CANISTER 10/19/2021 16 660 mL in 1 CANISTER; Type 0: Not a Combination Product 17 NDC:80948-019-17 1 in 1 PACKET 10/19/2021 17 6.6 mL in 1 PACKET; Type 0: Not a Combination Product 18 NDC:80948-019-18 25 in 1 BOX 10/19/2021 18 165 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 04/27/2021 Labeler - ZENITH MICRO CONTROL (915625571) Establishment Name Address ID/FEI Business Operations ZENITH MICRO CONTROL 915625571 manufacture(80948-019)