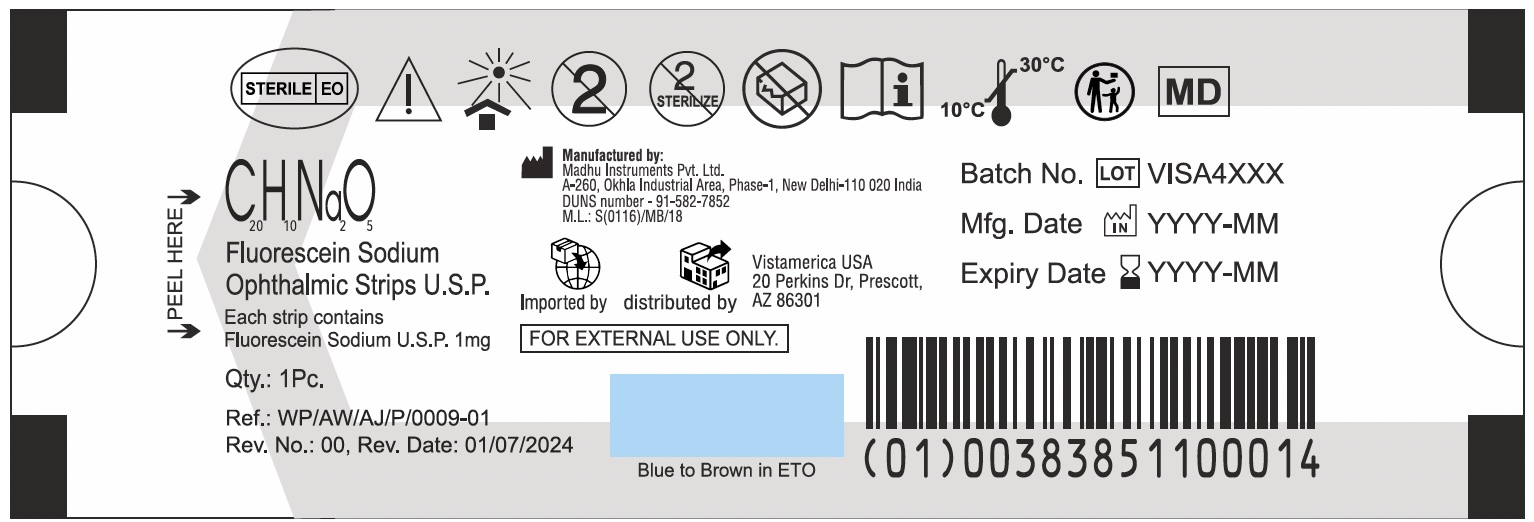
Label: C20H10NA2O5 FLUORESCEIN SODIUM OPHTHALMIC STRIPS- fluorescein sodium strip
- NDC Code(s): 83851-100-05, 83851-100-10, 83851-100-30
- Packager: Vistamerica USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Product Facts
- INDICATIONS:
-
DIRECTIONS FOR USE:
To insure full fluorescence and patient comfort, the impregnated tip should be moistened before application. One or two drops of sterile irrigating or saline solution should be used for this purpose. Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
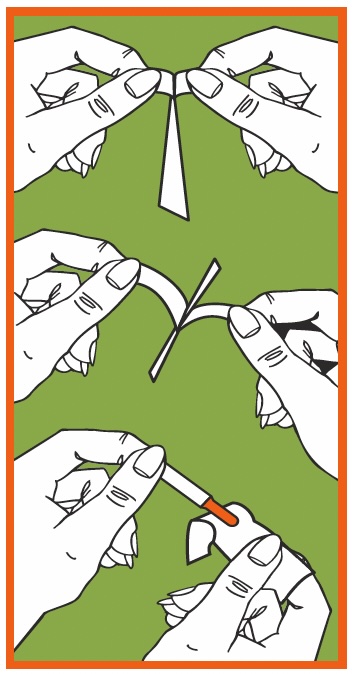
Grasp tabs between thumbs & index fingers
Gently pull tabs apart
Remove strip taking care to touch only the white portion of the strip
- STORAGE:
- NOTE:
- KEEP OUT OF REACH OF CHILDREN
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
- Representative Packaging:
-
INGREDIENTS AND APPEARANCE
C20H10NA2O5 FLUORESCEIN SODIUM OPHTHALMIC STRIPS
fluorescein sodium stripProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:83851-100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUORESCEIN SODIUM (UNII: 93X55PE38X) (FLUORESCEIN - UNII:TPY09G7XIR) FLUORESCEIN SODIUM 1 mg in 1 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83851-100-10 100 in 1 BOX 10/01/2024 1 1 mg in 1 PACKET; Type 0: Not a Combination Product 2 NDC:83851-100-30 300 in 1 BOX 10/01/2024 2 1 mg in 1 PACKET; Type 0: Not a Combination Product 3 NDC:83851-100-05 50 in 1 BOX 10/01/2024 3 1 mg in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2024 Labeler - Vistamerica USA (122342378) Establishment Name Address ID/FEI Business Operations Madhu Instruments Pvt.Ltd. 915827852 manufacture(83851-100)




 83851-100: Fluorescein Sodium Ophthalmic Strip Box (above)
83851-100: Fluorescein Sodium Ophthalmic Strip Box (above)