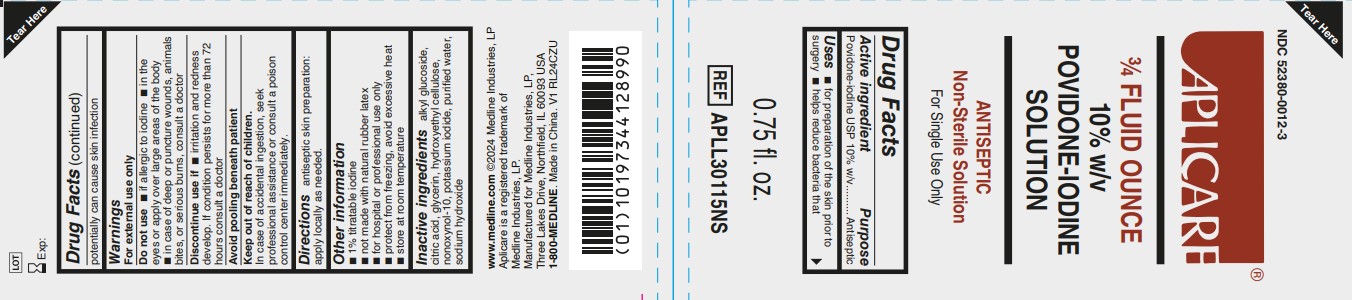
Label: APLICARE- povidone-iodine solution
- NDC Code(s): 52380-0012-3
- Packager: Aplicare Products, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 12, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- if allergic to iodine
- in the eyes or apply over large areas of the body
- in case of deep or puncture wounds, animals bites, or serious burns, consult a doctor
- Directions
- Other information
- Inactive ingredients
- Manufacturing information
- Package Label
-
INGREDIENTS AND APPEARANCE
APLICARE
povidone-iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52380-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-10 (UNII: K7O76887AP) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID (UNII: 2968PHW8QP) POTASSIUM IODIDE (UNII: 1C4QK22F9J) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52380-0012-3 22.5 mL in 1 PACKET; Type 0: Not a Combination Product 12/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/01/2024 Labeler - Aplicare Products, LLC (081054904) Registrant - Medline Industries, LP (025460908)