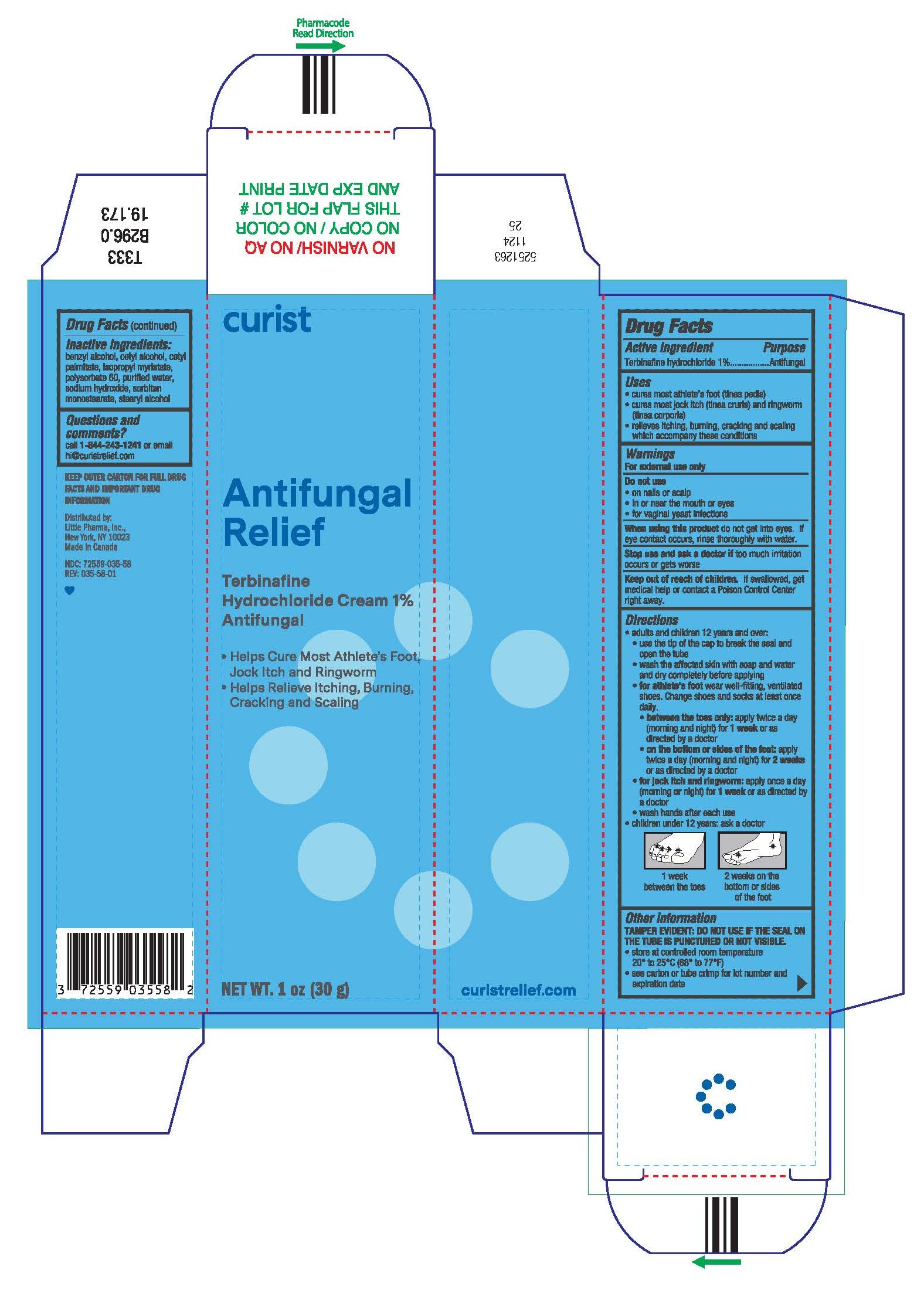
Label: CURIST ANTIFUNGAL RELIEF- terbinafine hydrochloride cream
- NDC Code(s): 72559-035-58
- Packager: Little Pharma, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 10, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- adults and children 12 years and older:
- use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying
-
for athlete's foot wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
- between the toes only:apply twice a day (morning and night) for 1 week or as directed by a doctor.
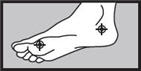
- on the bottom or sides of the foot:apply twice a day (morning and night) for 2 weeks or as directed by a doctor.
- for jock itch and ringworm:apply once a day (morning ornight) for 1 week or as directed by a doctor.
- wash hands after each use
- children under 12 years: ask a doctor
1 week between the toes

2 weeks on the bottom or sides of the foot
- adults and children 12 years and older:
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 g Tube Carton
-
INGREDIENTS AND APPEARANCE
CURIST ANTIFUNGAL RELIEF
terbinafine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72559-035 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TERBINAFINE HYDROCHLORIDE (UNII: 012C11ZU6G) (TERBINAFINE - UNII:G7RIW8S0XP) TERBINAFINE 1 g in 100 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CETYL ALCOHOL (UNII: 936JST6JCN) CETYL PALMITATE (UNII: 5ZA2S6B08X) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POLYSORBATE 60 (UNII: CAL22UVI4M) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72559-035-58 1 in 1 CARTON 12/10/2024 1 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077511 12/10/2024 Labeler - Little Pharma, Inc. (074328189) Establishment Name Address ID/FEI Business Operations Taro Pharmaceuticals Inc. 206263295 manufacture(72559-035)