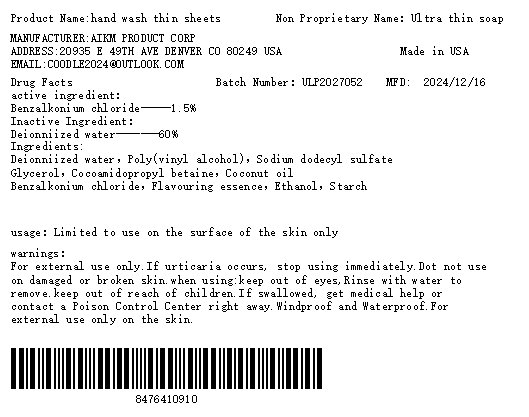
Label: HAND WASH THIN SHEETS- ultra thin soap soap
- NDC Code(s): 84764-109-10, 84764-109-20, 84764-109-50
- Packager: AIKM Product Corp
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 16, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- active ingredient
- Purpose
- usage
- warnings
- stop
- do not use
- when using
- keep out of reach of children
- storage
- For external use only on the skin
- INACTIVE INGREDIENT
- Use according to the instructions or doctor's advice
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HAND WASH THIN SHEETS
ultra thin soap soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84764-109 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 100 mg in 1 g Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84764-109-20 20 g in 1 PACKAGE; Type 0: Not a Combination Product 12/16/2024 2 NDC:84764-109-10 10 g in 1 PACKAGE; Type 0: Not a Combination Product 12/16/2024 3 NDC:84764-109-50 50 g in 1 PACKAGE; Type 0: Not a Combination Product 12/16/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M031 12/16/2024 Labeler - AIKM Product Corp (131889830)