Label: GUAIFENESIN AND PSEUDOEPHEDRINE HCL tablet, extended release
- NDC Code(s): 70677-1263-1
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 28, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
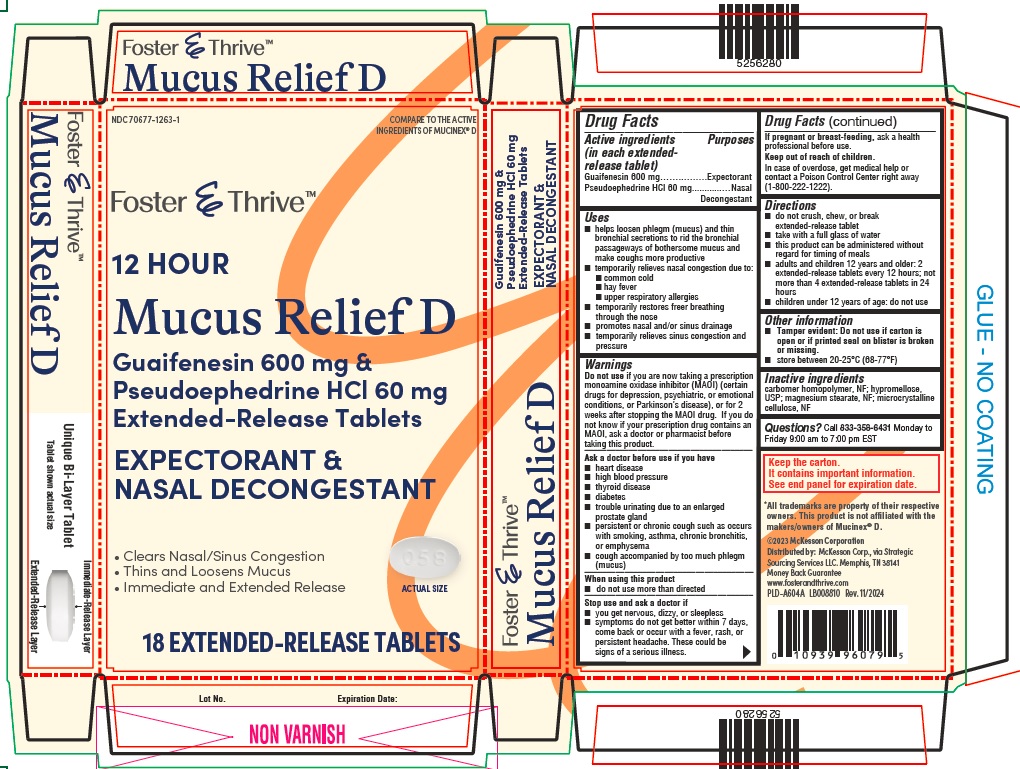
- ACTIVE INGREDIENT
-
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
- temporarily relieves sinus congestion and pressure
-
Warnings
Do not useif you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
When using this product
- do not use more than directed
Stop use and ask a doctor if
- you get nervous, dizzy, or sleepless
- symptoms do not get better within 7 days, come back or occur with a fever, rash, or persistent headache. These could be signs of a serious illness.
If pregnant or breast-feeding, ask a health professional before use.
-
Directions
- do not crush, chew, or break extended-release tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years and older: 2 extended-release tablets every 12 hours; not more than 4 extended-release tablets in 24 hours
- children under 12 years of age: do not use
- Inactive Ingredients
-
Questions?
Call 833-358-6431 Monday to Friday 9:00 am to 7:00 pm EST
Keep the carton. It contains important information.
See end panel for expiration date.
*All trademarks are property of their respective owners. This product is not affliated with the makers/owners of Mucinex® D.
©2023 McKesson Corporation
Distributed by: McKesson Corp.,via Strategic Sourcing Services LLC.
Memphis, TN 38141
Money Back Guarantee
www.fosterandthrive.com
Rev. 11/2024 -
Guaifenesin 600 mg and Pseudoephedrine HCl 60 mg Extended-Release Tablets - Carton Label
NDC 70677-1263-1
COMPARE TO THE ACTIVE
INGREDIENTS OF MUCINEX ®D
Foster & Thrive TM
12 HOUR
Mucus Relief D
Guaifenesin 600 mg & Pseudoephedrine HCl 60 mg
Extended-Release TabletsEXPECTORANT & NASAL DECONGESTANT
- Clears Nasal/Sinus Congestion
- Thins and Loosens Mucus
- Immediate and Extended Release
ACTUAL SIZE
18 EXTENDED-RELEASE TABLETS
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND PSEUDOEPHEDRINE HCL
guaifenesin and pseudoephedrine hcl tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70677-1263 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white (white to off-white) Score no score Shape OVAL Size 17mm Flavor Imprint Code 058 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70677-1263-1 1 in 1 CARTON 12/13/2024 1 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212542 12/13/2024 Labeler - Strategic Sourcing Services LLC (116956644) Establishment Name Address ID/FEI Business Operations Ohm Laboratories, Inc. 184769029 manufacture(70677-1263)

