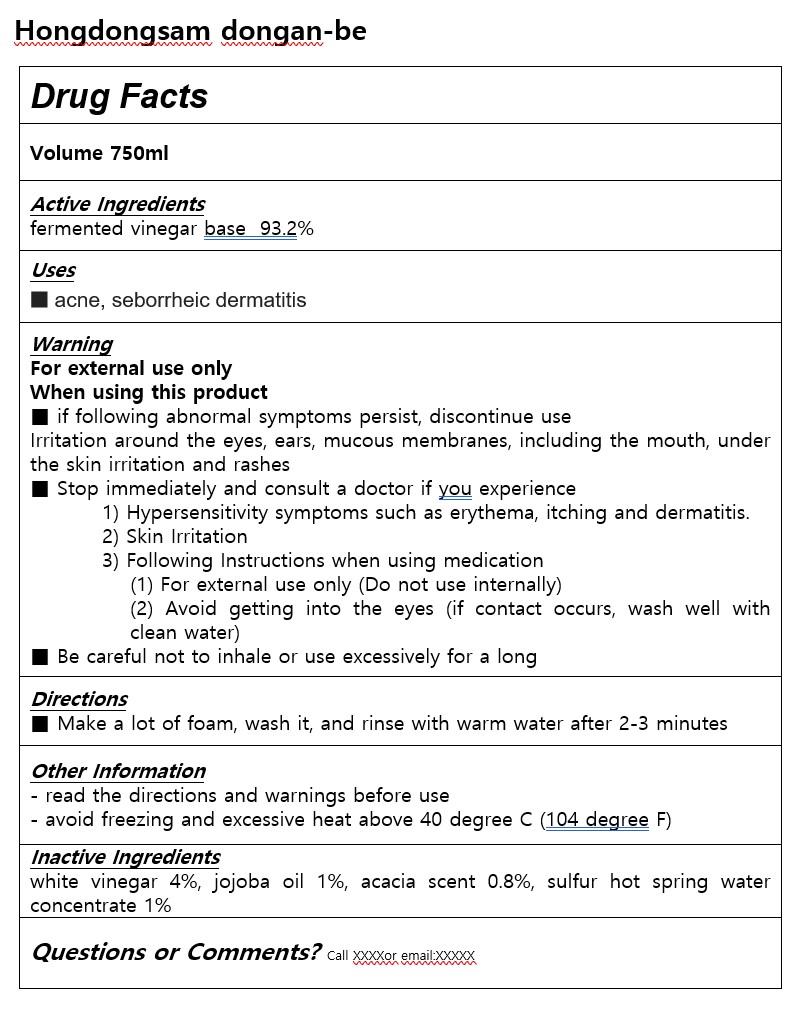
Label: HONGDONGSAM DONGAN-BE- fermented vinegar base soap
- NDC Code(s): 84954-0002-1
- Packager: Hyosungwon
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
For external use only
When using this product
■ if following abnormal symptoms persist, discontinue use
Irritation around the eyes, ears, mucous membranes, including the mouth, under the skin irritation and rashes
■ Stop immediately and consult a doctor if you experience
1) Hypersensitivity symptoms such as erythema, itching and dermatitis.
2) Skin Irritation
3) Following Instructions when using medication
(1) For external use only (Do not use internally)
(2) Avoid getting into the eyes (if contact occurs, wash well with clean water)
■ Be careful not to inhale or use excessively for a long
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HONGDONGSAM DONGAN-BE
fermented vinegar base soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84954-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APPLE CIDER VINEGAR (UNII: 0UE22Q87VC) (APPLE CIDER VINEGAR - UNII:0UE22Q87VC) APPLE CIDER VINEGAR 93.2 g in 100 mL Inactive Ingredients Ingredient Name Strength JOJOBA OIL (UNII: 724GKU717M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84954-0002-1 750 mL in 1 PACKAGE; Type 0: Not a Combination Product 11/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/10/2024 Labeler - Hyosungwon (987424933) Registrant - Hyosungwon (987424933) Establishment Name Address ID/FEI Business Operations Agricultural Corporation YUBIMUHUAN CO Ltd 695152747 manufacture(84954-0002)