Label: FUNGIFREE FUNGAL NAIL PATCHES- tolnaftate patch
- NDC Code(s): 83364-013-01
- Packager: YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATION
Directions
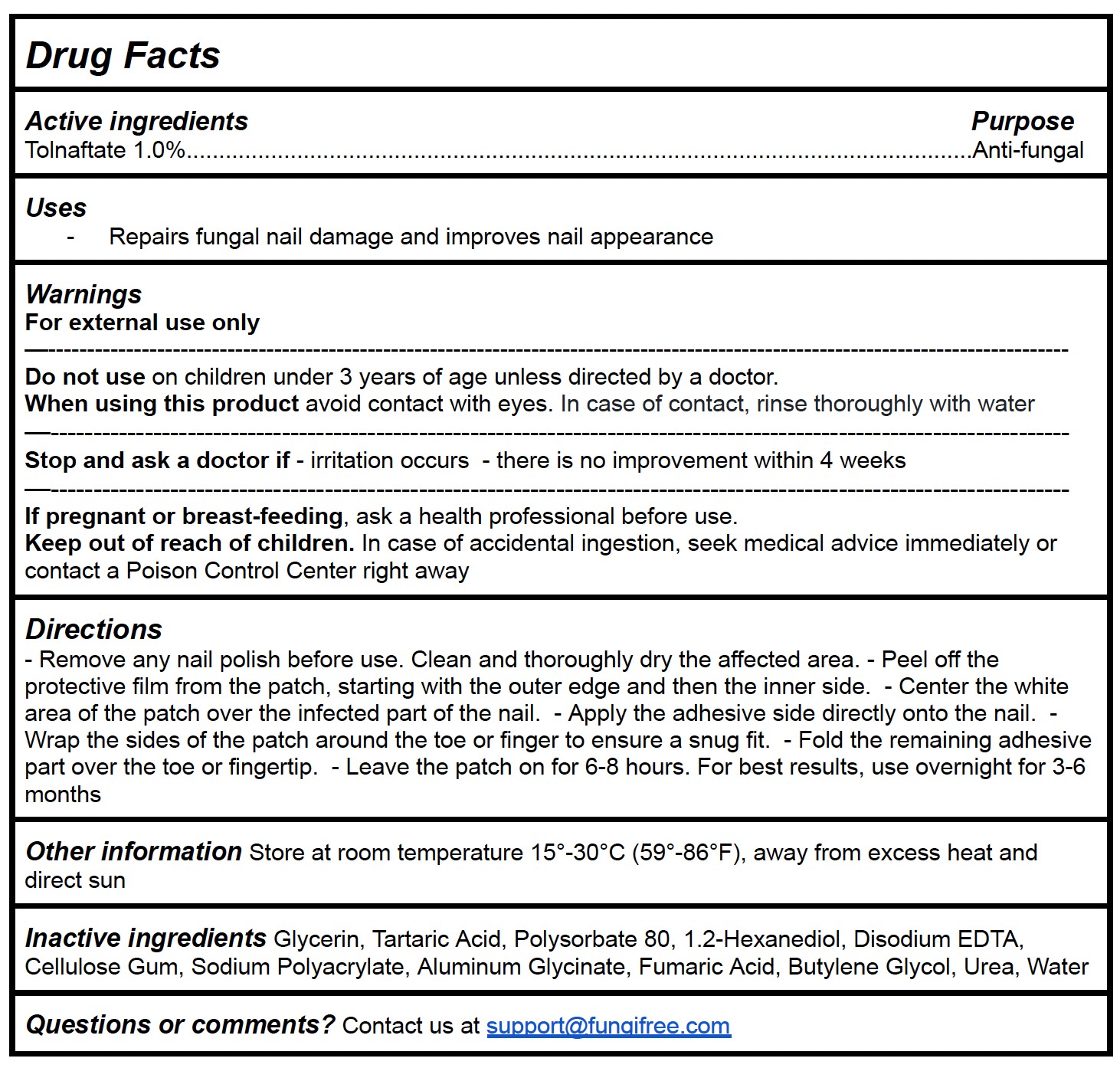
- Remove any nail polish before use. Clean and thoroughly dry the affected area. - Peel off the protective film from the patch, starting with the outer edge and then the inner side. - Center the white area of the patch over the infected part of the nail. - Apply the adhesive side directly onto the nail. - Wrap the sides of the patch around the toe or finger to ensure a snug fit. - Fold the remaining adhesive part over the toe or fingertip. - Leave the patch on for 6-8 hours. For best results, use overnight for 3-6 months
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FUNGIFREE FUNGAL NAIL PATCHES
tolnaftate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83364-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM GLYCINATE (UNII: 1K713C615K) CELLULOSE GUM (UNII: K679OBS311) TARTARIC ACID (UNII: W4888I119H) EDETATE DISODIUM (UNII: 7FLD91C86K) UREA (UNII: 8W8T17847W) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) FUMARIC ACID (UNII: 88XHZ13131) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83364-013-01 21 in 1 PATCH 10/21/2024 1 0.7 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/21/2024 Labeler - YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD (725220463)