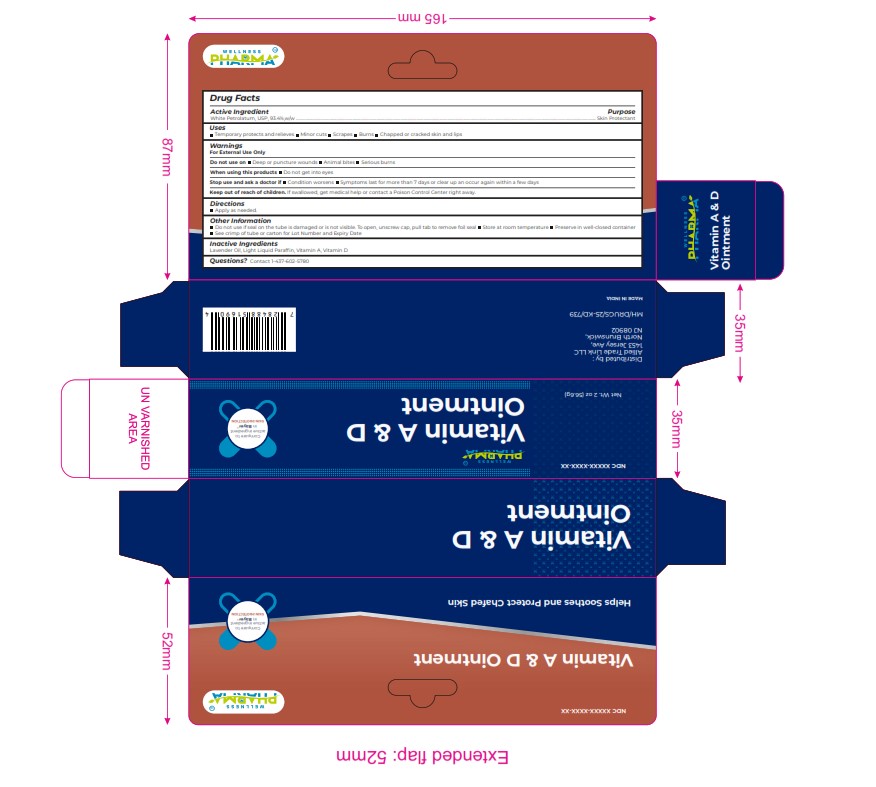
Label: VITAMIN A AND D ointment
- NDC Code(s): 84387-007-01
- Packager: ALLIED TRADE LINK LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 13, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
-
WARNINGS AND PRECAUTIONS
Warnings
For External Use Only
Do not use on Deep or puncture wounds Animal bites Serious burns
When using this products Do not get into eyes
Stop use and ask a doctor if Condition worsens Symptoms last for more than 7 days or clear up an occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
-
WARNINGS
Warnings
For External Use Only
Do not use on Deep or puncture wounds Animal bites Serious burns
When using this products Do not get into eyes
Stop use and ask a doctor if Condition worsens Symptoms last for more than 7 days or clear up an occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN A AND D
vitamin a and d ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84387-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) (WHITE PETROLATUM - UNII:B6E5W8RQJ4) WHITE PETROLATUM 93.4 g in 100 g Inactive Ingredients Ingredient Name Strength PARAFFINUM LIQUIDUM (UNII: T5L8T28FGP) LAVENDER OIL (UNII: ZBP1YXW0H8) VITAMIN D (UNII: 9VU1KI44GP) VITAMIN A (UNII: 81G40H8B0T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84387-007-01 56.6 g in 1 TUBE; Type 0: Not a Combination Product 11/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/13/2024 Labeler - ALLIED TRADE LINK LLC (029033682) Registrant - ALLIED TRADE LINK LLC (029033682) Establishment Name Address ID/FEI Business Operations GOLPALDAS VISRAM AND COMPANY LIMITED 858030888 manufacture(84387-007)