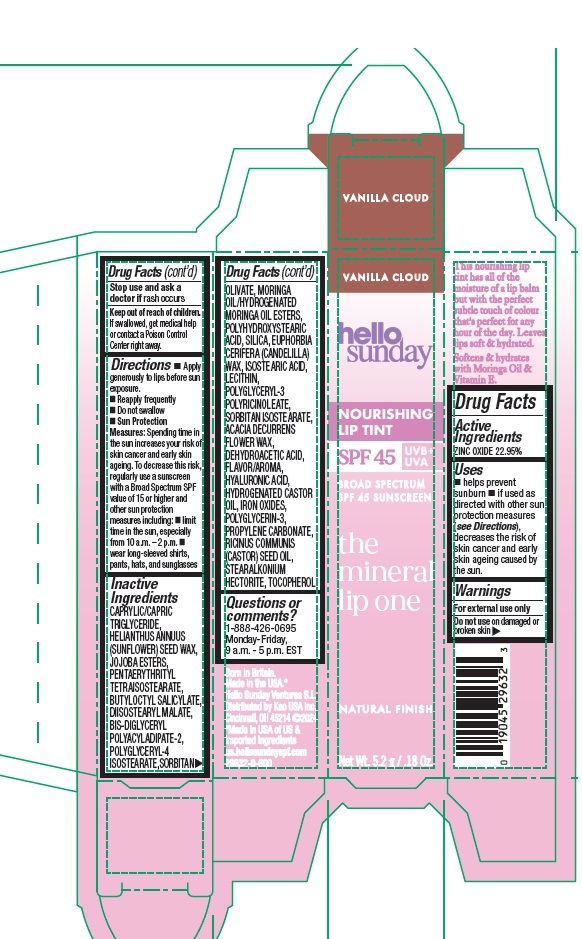
Label: HELLO SUNDAY THE MINERAL LIP ONE NOURISHING LIP TINT SPF 45 VANILLA CLOUD- zinc oxide stick
- NDC Code(s): 10596-002-01
- Packager: Kao USA Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 13, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply generously to lips before sun exposure.
- Reapply frequently
- Do not swallow
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin ageing. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
-
Inactive Ingredients
CAPRYLIC/CAPRIC TRIGLYCERIDE, HELIANTHUS ANNUUS (SUNFLOWER) SEED WAX, JOJOBA ESTERS, PENTAERYTHRITYL TETRAISOSTEARATE, DIISOSTEARYL MALATE, BUTYLOCTYL SALICYLATE, BIS-DIGLYCERYL POLYACYLADIPATE-2, POLYGLYCERYL-4 ISOSTEARATE, SORBITAN OLIVATE, MORINGA OIL/HYDROGENATED MORINGA OIL ESTERS, POLYHYDROXYSTEARIC ACID, SILICA, EUPHORBIA CERIFERA (CANDELILLA) WAX, ISOSTEARIC ACID, LECITHIN, POLYGLYCERYL-3 POLYRICINOLEATE, SORBITAN ISOSTEARATE, ACACIA DECURRENS FLOWER WAX, DEHYDROACETIC ACID, FLAVOR/AROMA, HYALURONIC ACID, HYDROGENATED CASTOR OIL, IRON OXIDES, POLYGLYCERIN-3, PROPYLENE CARBONATE, RICINUS COMMUNIS (CASTOR) SEED OIL, STEARALKONIUM HECTORITE, TOCOPHEROL
- Questions or comments?
- Product Packaging
-
INGREDIENTS AND APPEARANCE
HELLO SUNDAY THE MINERAL LIP ONE NOURISHING LIP TINT SPF 45 VANILLA CLOUD
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10596-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 229.5 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) HYALURONIC ACID (UNII: S270N0TRQY) IRON OXIDES (UNII: 1K09F3G675) PROPYLENE CARBONATE (UNII: 8D08K3S51E) CASTOR OIL (UNII: D5340Y2I9G) HELIANTHUS ANNUUS (SUNFLOWER) SEED WAX (UNII: 42DG15CHXV) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) SORBITAN OLIVATE (UNII: MDL271E3GR) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DEHYDROACETIC ACID (UNII: 2KAG279R6R) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) ISOSTEARIC ACID (UNII: X33R8U0062) PENTAERYTHRITYL TETRAISOSTEARATE (UNII: 9D7IK5483F) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) TOCOPHEROL (UNII: R0ZB2556P8) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CANDELILLA WAX (UNII: WL0328HX19) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) CAPRYLIC/CAPRIC TRIGLYCERIDE (UNII: C9H2L21V7U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10596-002-01 1 in 1 CARTON 11/12/2024 1 5.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/12/2024 Labeler - Kao USA Inc. (004251617)