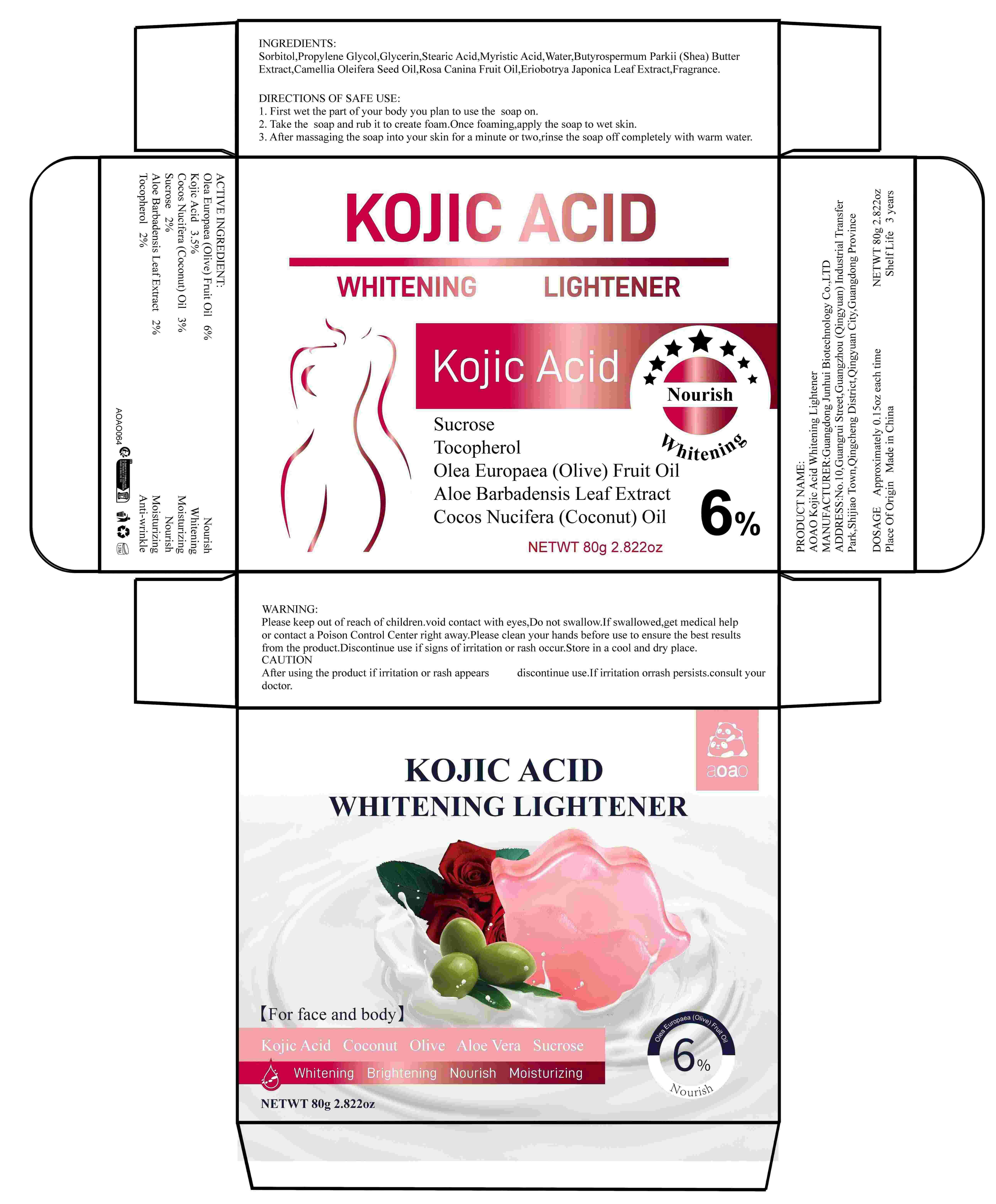
Label: AOAO KOJIC ACID WHITENING LIGHTENER (olea europaea (olive) fruit oil,kojic acid,cocos nucifera- coconut oil,sucrose,aloe barbadensis leaf extract,tocopherol soap
- NDC Code(s): 84509-049-01
- Packager: Guangdong Junhui Biotechnology Co., LTD
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 12, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
-
Uses
DIRECTIONS OF SAFE USE:
1. First wet the part of your body you plan to use the exfoliating soap on.
2. Take the exfoliating soap and rub it to create foam. Once foaming, apply the soap to wet skin.
3. After massaging the soap into your skin for a minute or two, rinse the soap off completely with warm water.
- Warnings
- Keep out of reach of children
- CAUTION
- Inactive Ingredients
- Product Name
- DOSAGE
- Manufacturer
- ADDRESS
- Capacity
- Shelf Life
- Place of Origin
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AOAO KOJIC ACID WHITENING LIGHTENER
olea europaea (olive) fruit oil,kojic acid,cocos nucifera (coconut) oil,sucrose,aloe barbadensis leaf extract,tocopherol soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84509-049 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLEA EUROPAEA (OLIVE) FRUIT OIL (UNII: 6UYK2W1W1E) (OLEA EUROPAEA (OLIVE) FRUIT OIL - UNII:6UYK2W1W1E) OLEA EUROPAEA (OLIVE) FRUIT OIL 4800 mg in 80 g SUCROSE (UNII: C151H8M554) (SUCROSE - UNII:C151H8M554) SUCROSE 1600 mg in 80 g COCOS NUCIFERA (COCONUT) OIL (UNII: Q9L0O73W7L) (COCOS NUCIFERA (COCONUT) OIL - UNII:Q9L0O73W7L) COCOS NUCIFERA (COCONUT) OIL 2400 mg in 80 g KOJIC ACID (UNII: 6K23F1TT52) (KOJIC ACID - UNII:6K23F1TT52) KOJIC ACID 2800 mg in 80 g ALOE BARBADENSIS LEAF EXTRACT (UNII: ZY81Z83H0X) (ALOE BARBADENSIS LEAF EXTRACT - UNII:ZY81Z83H0X) ALOE BARBADENSIS LEAF EXTRACT 1600 mg in 80 g TOCOPHEROL (UNII: R0ZB2556P8) (TOCOPHEROL - UNII:R0ZB2556P8) TOCOPHEROL 1600 mg in 80 g Inactive Ingredients Ingredient Name Strength MYRISTIC ACID (UNII: 0I3V7S25AW) CAMELLIA OLEIFERA SEED OIL (UNII: T1PE06G0VE) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ROSA CANINA FRUIT OIL (UNII: CR7307M3QZ) FRAGRANCE 13576 (UNII: 5EM498GW35) ERIOBOTRYA JAPONICA LEAF EXTRACT (UNII: Z02066SV11) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) SORBITOL (UNII: 506T60A25R) BUTYROSPERMUM PARKII (SHEA) BUTTER EXTRACT (UNII: K49155WL9Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84509-049-01 80 g in 1 BOX; Type 0: Not a Combination Product 11/12/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/12/2024 Labeler - Guangdong Junhui Biotechnology Co., LTD (707735253) Registrant - Dongguan Xiaogoubeige trading company Ltd. (707735253) Establishment Name Address ID/FEI Business Operations Dongguan Xiaogoubeige trading company Ltd. 707735253 label(84509-049) , manufacture(84509-049)