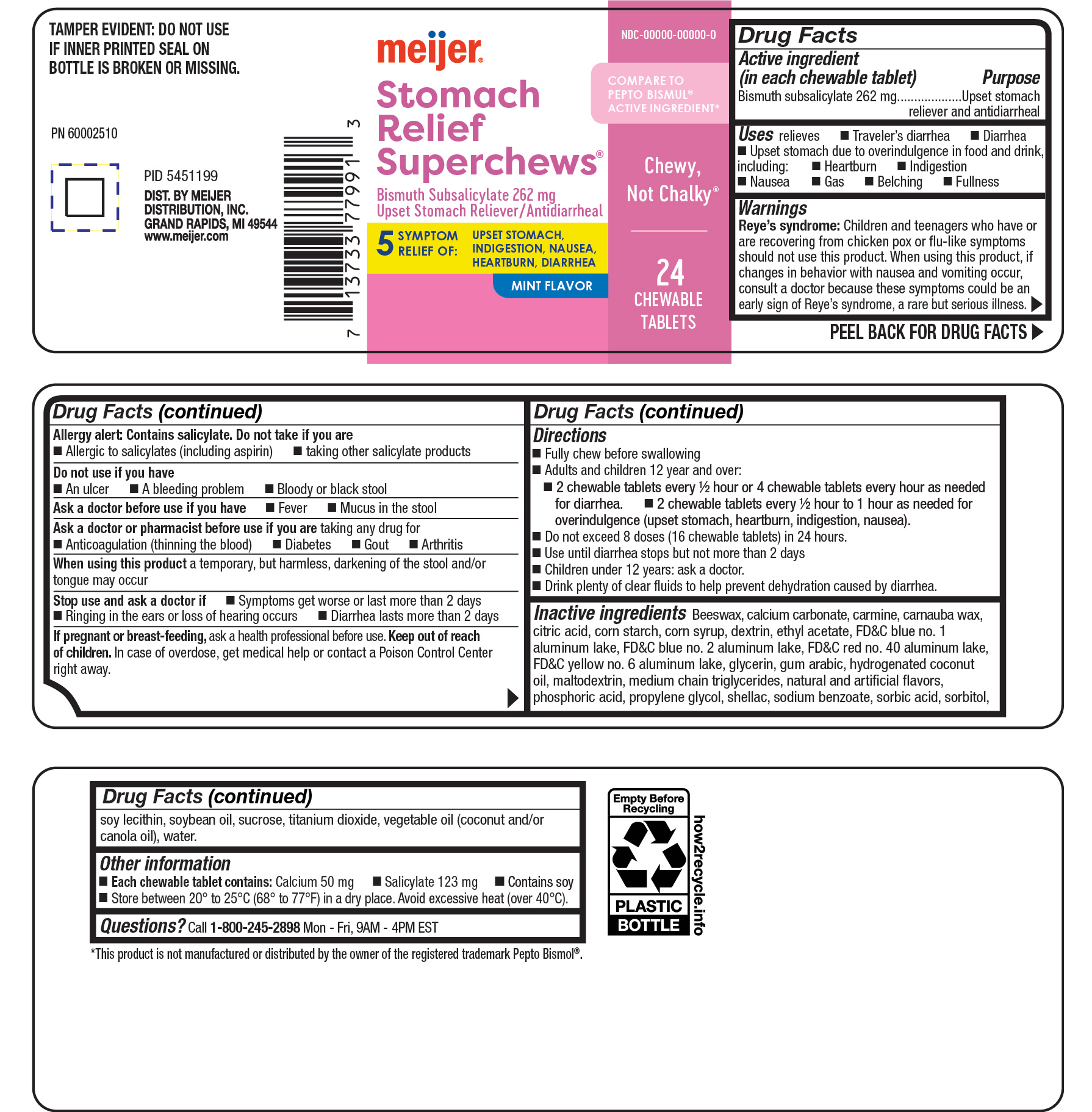
Label: MEIJER BISMUTH STOMACH RELIEF- bismuth tablet, chewable
- NDC Code(s): 79481-0097-1
- Packager: Meijer
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- Allergic to salicylates (including aspirin)
- taking other salicylates products
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
-
DOSAGE & ADMINISTRATION
Directions
- Fully chew before swallowing
- Adults and children 12 years abd over:
- 2 chewable tablets every 1/2 hour or 4 chewable tablets every hour as needed for diarrhea.
- 2 chewable tablets every 1/2 hour to 1 hour as needed for overindulgence (upset stomach, heartburn, indigenstion, nausea).
- Do not exceed 8 doses (16 chewable tablets) in 24 hours.
- Use until diarrhea stops but not more than 2 days
- Children under 12 years: ask a doctor.
- Drink plenty of clear fluids to help prevent dehydration caused by diarrhea.
-
INACTIVE INGREDIENT
Inactive ingredients: Beeswax, calcium carbonate, carmine, carnauba wax, citric acid, corn starch, corn syrup, dextrin, ethyl acetate, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, FD&C yellow no. 6 aluminum lake, glycerin, gum arabic, hydrogenated coconut oil, maltodextrin, medium chain triglycerides, natural and artificial flavors, phosphoric acid, propylene glycol, shellac, sodium benzoate, sorbic acid, sorbitol, soy lecithin, soybean oil, sucrose, titanium dioxide, vegetable oil (coconut and/or canola oil), water.
- OTHER SAFETY INFORMATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEIJER BISMUTH STOMACH RELIEF
bismuth tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-0097 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (BISMUTH CATION - UNII:ZS9CD1I8YE) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) Product Characteristics Color pink Score no score Shape ROUND Size 14mm Flavor MINT Imprint Code SR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-0097-1 24 in 1 BOTTLE; Type 0: Not a Combination Product 11/11/2024 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M008 11/11/2024 Labeler - Meijer (006959555) Registrant - Bestco (002149136) Establishment Name Address ID/FEI Business Operations Bestco 002149136 manufacture(79481-0097)