Label: KUMARY- k-umary tablet
- NDC Code(s): 84858-1010-1, 84858-1010-2
- Packager: METAZONKEY LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 5, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
-
WARNINGS
 WARNING: If you are pregnant, nursing, taking any medicationsor planning any medical procedure, consult your physician before use. Discontinue use and consult your physician if any adverse reactions occour. Keep out of reach of children.
WARNING: If you are pregnant, nursing, taking any medicationsor planning any medical procedure, consult your physician before use. Discontinue use and consult your physician if any adverse reactions occour. Keep out of reach of children.
Store in dry place under controlled room temperature: 20 - 25 °C
(68-77°F) - INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KUMARY
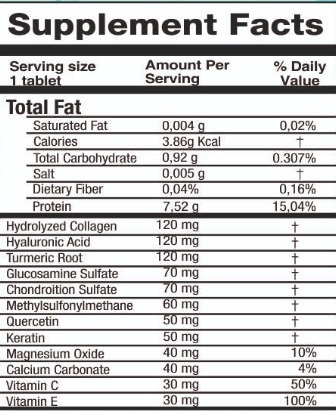
k-umary tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84858-1010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHONDROITIN SULFATE (BOVINE) (UNII: 6IC1M3OG5Z) (CONDOLIASE - UNII:7SI2UZG934) CONDOLIASE 70 mg in 1 U HYDROLYZED SHEEP WOOL KERATIN (200 MW) (UNII: R6K7AW17SU) (HYDROLYZED SHEEP WOOL KERATIN (200 MW) - UNII:R6K7AW17SU) HYDROLYZED SHEEP WOOL KERATIN (200 MW) 50 mg in 1 U HYALURONIC ACID (UNII: S270N0TRQY) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONIC ACID 120 mg in 1 U CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 40 mg in 1 U TURMERIC (UNII: 856YO1Z64F) (TURMERIC - UNII:856YO1Z64F) TURMERIC 120 mg in 1 U COLLAGEN ALPHA-2(I) CHAIN BOVINE (UNII: YRU8Z30SXI) (COLLAGEN ALPHA-2(I) CHAIN BOVINE - UNII:YRU8Z30SXI) COLLAGEN ALPHA-2(I) CHAIN BOVINE 120 mg in 1 U MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 40 mg in 1 U POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 100 mg in 1 U MAGNESIUM STEARATE (UNII: 70097M6I30) (MAGNESIUM STEARATE - UNII:70097M6I30) MAGNESIUM STEARATE 10 mg in 1 U ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 30 mg in 1 U Inactive Ingredients Ingredient Name Strength GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) 70 mg in 1 U .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) 30 mg in 1 U CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) 340 mg in 1 U Product Characteristics Color yellow Score no score Shape CAPSULE Size 10mm Flavor GINGER Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84858-1010-1 30 U in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/06/2024 2 NDC:84858-1010-2 30 U in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/06/2024 10/01/2026 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/05/2024 Labeler - METAZONKEY LLC (119340119)


 For adults take 1 tablet with water daily
For adults take 1 tablet with water daily