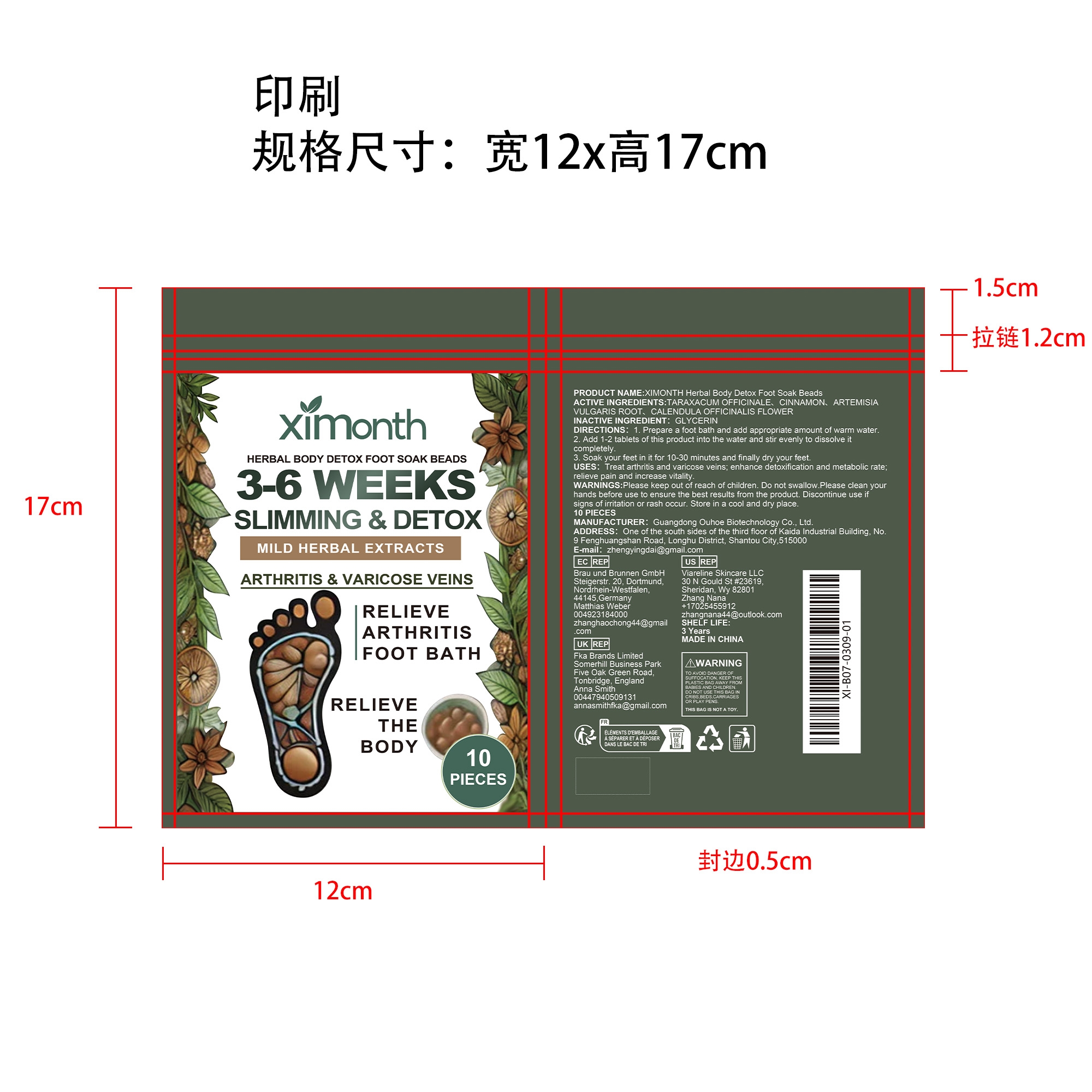
Label: XIMONTH HERBAL BODY DETOX FOOT SOAK BEADS- glycerin patch
- NDC Code(s): 84744-070-01
- Packager: Guangdong Ouhoe Biotechnology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 28, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
-
PURPOSE
Treat arthritis and varicose veins; enhance detoxification and metabolic rate;relieve pain and increase vitality. WARNINGS:Please keep out of reach of children. Do not swallow.Please clean yourhands before use to ensure the best results from the product. Discontinue use ifsigns of irritation or rash occur. Store in a cool and dry place.
- USE
- WARNINGS
- STOP USE
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
XIMONTH HERBAL BODY DETOX FOOT SOAK BEADS
glycerin patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84744-070 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 3 g in 10 g ARTEMISIA VULGARIS ROOT (UNII: 32MP823R8S) (ARTEMISIA VULGARIS ROOT - UNII:32MP823R8S) ARTEMISIA VULGARIS ROOT 1.5 g in 10 g CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) (CALENDULA OFFICINALIS FLOWER - UNII:P0M7O4Y7YD) CALENDULA OFFICINALIS FLOWER 1.5 g in 10 g CINNAMON (UNII: 5S29HWU6QB) (CINNAMON - UNII:5S29HWU6QB) CINNAMON 1 g in 10 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 3 g in 10 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84744-070-01 10 g in 1 PACKAGE; Type 0: Not a Combination Product 10/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 10/29/2024 Labeler - Guangdong Ouhoe Biotechnology Co., Ltd. (843651433) Registrant - Guangdong Ouhoe Biotechnology Co., Ltd. (843651433) Establishment Name Address ID/FEI Business Operations Guangdong Ouhoe Biotechnology Co., Ltd. 843651433 manufacture(84744-070)