Label: SENNA LAXATIVE- sennosides tablet
- NDC Code(s): 41163-782-10
- Packager: United Natural Foods, Inc. dba UNFI
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 23, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
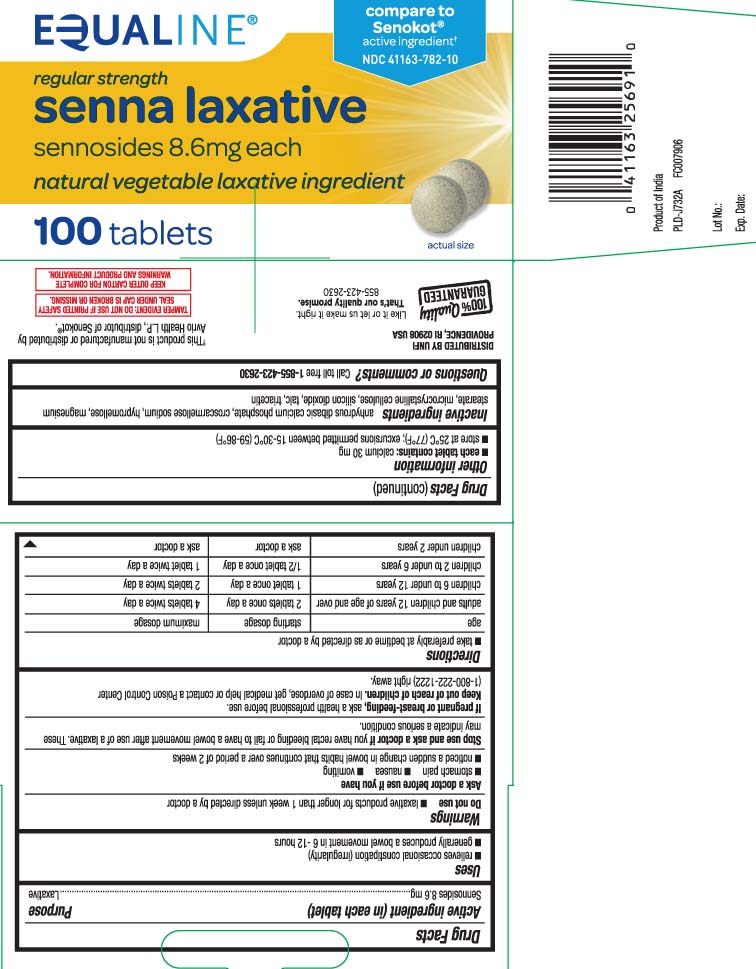
- Active ingredients (in each tablet)
- Purpose
- Uses
- Warnings
-
Directions
- take preferably at bedtime or as directed by a doctor
age starting dosage maximum dosage adults and children 12 years of age and over 2 tablets once a day 4 tablets twice a day children 6 to under 12 years 1 tablet once a day 2 tablets twice a day children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day children under 2 years ask a doctor ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
compare to Senokot® active ingredient†
regular strength
senna laxative
sennosides 8.6 mg each
natural vegetable laxative ingredient
tablets
†This product is not manufactured ir distributed by Avrio Health L.P., distributor of Senokot®.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
DISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA
- Product Labeling
-
INGREDIENTS AND APPEARANCE
SENNA LAXATIVE
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41163-782 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code PS23 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41163-782-10 1 in 1 BOX 10/29/2021 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 10/29/2021 Labeler - United Natural Foods, Inc. dba UNFI (943556183)