Label: PFECT A SOLAR BARRIER SERUM TEXTURED- pfect-a solar barrier elixir serum textured elixir
- NDC Code(s): 84755-0002-1
- Packager: KRX RESOURCES SDN. BHD.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 22, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- WARNINGS
-
INACTIVE INGREDIENT
Inactive Ingredients:
WATER, PROPANEDIOL, DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE, POLYMETHYLSILSESQUIOXANE, ETHYLHEXYL TRIAZONE, NIACINAMIDE, METHYLENE BIS- BENZOTRIAZOLYL TETRAMETHYLBUTYLPHENOL, COCO- CAPRYLATE/CAPRATE, CAPRYLYL METHICONE, DIETHYLHEXYL BUTAMIDO TRIAZONE, GLYCERIN, SODIUM HYALURONATE, BUTYLENE GLYCOL, CAMELLIA SINENSIS LEAF EXTRACT, SACCHARUM OFFICINARUM (SUGARCANE) EXTRACT, ACETYL HEXAPEPTIDE-8, MACROCYSTIS PYRIFERA (KELP) EXTRACT, COCOS NUCIFERA (COCONUT) FRUIT EXTRACT, PANAX GINSENG ROOT EXTRACT, PANTHENOL, PENTYLENE GLYCOL, POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE, DECYL GLUCOSIDE, TROMETHAMINE, CARBOMER, 1,2- HEXANEDIOL,SODIUM STEAROYL GLUTAMATE, POLYACRYLATE CROSSPOLYMER-6, CARBOMER, ETHYLHEXYLGLYCERIN, ADENOSINE, XANTHAN GUM, GLUTATHIONE, TOCOPHEROL. - INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
-
PURPOSE
•Nourishing •Anti Ageing •Sun Protection
Non Sticky and No white cast SPF 50 PA ++++ Suncreen formula, with UVA and UVB protection. Rich in Vitamins, Peptides and Nutrients for both hydration, anti ageing and sun protection. Suitable for all skin types. Directions: Apply liberally and spread evenly by hand before sun exposure.
- ACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PFECT A SOLAR BARRIER SERUM TEXTURED
pfect-a solar barrier elixir serum textured elixirProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84755-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETHYLHEXYL TRIAZONE (UNII: XQN8R9SAK4) (ETHYLHEXYL TRIAZONE - UNII:XQN8R9SAK4) ETHYLHEXYL TRIAZONE 1.5 mg in 50 mL DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) (DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE - UNII:ANQ870JD20) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE 2.5 mg in 50 mL Inactive Ingredients Ingredient Name Strength CARBOMER 934 (UNII: Z135WT9208) POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE (UNII: W19EIO0DBE) WATER (UNII: 059QF0KO0R) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) PEG-9 DIGLYCIDYL ETHER/SODIUM HYALURONATE CROSSPOLYMER (UNII: 788QAG3W8A) CARBOMER 980 (UNII: 4Q93RCW27E) GLUTATHIONE (UNII: GAN16C9B8O) TOCOPHEROL (UNII: R0ZB2556P8) ADENOSINE (UNII: K72T3FS567) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYL CAPRYLATE/CAPRATE (UNII: 7RXW88PS4S) NIACINAMIDE (UNII: 25X51I8RD4) PANTHENOL (UNII: WV9CM0O67Z) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) TROMETHAMINE (UNII: 023C2WHX2V) SACCHARUM OFFICINARUM STEM FRUCTOOLIGOSACCHARIDES (UNII: 8LLD82AE3S) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) PENTYLENE GLYCOL (UNII: 50C1307PZG) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPANEDIOL (UNII: 5965N8W85T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84755-0002-1 50 mL in 1 PACKAGE; Type 0: Not a Combination Product 09/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2024 Labeler - KRX RESOURCES SDN. BHD. (813058498) Registrant - KRX RESOURCES SDN. BHD. (813058498) Establishment Name Address ID/FEI Business Operations KRX RESOURCES SDN. BHD. 813058498 manufacture(84755-0002)


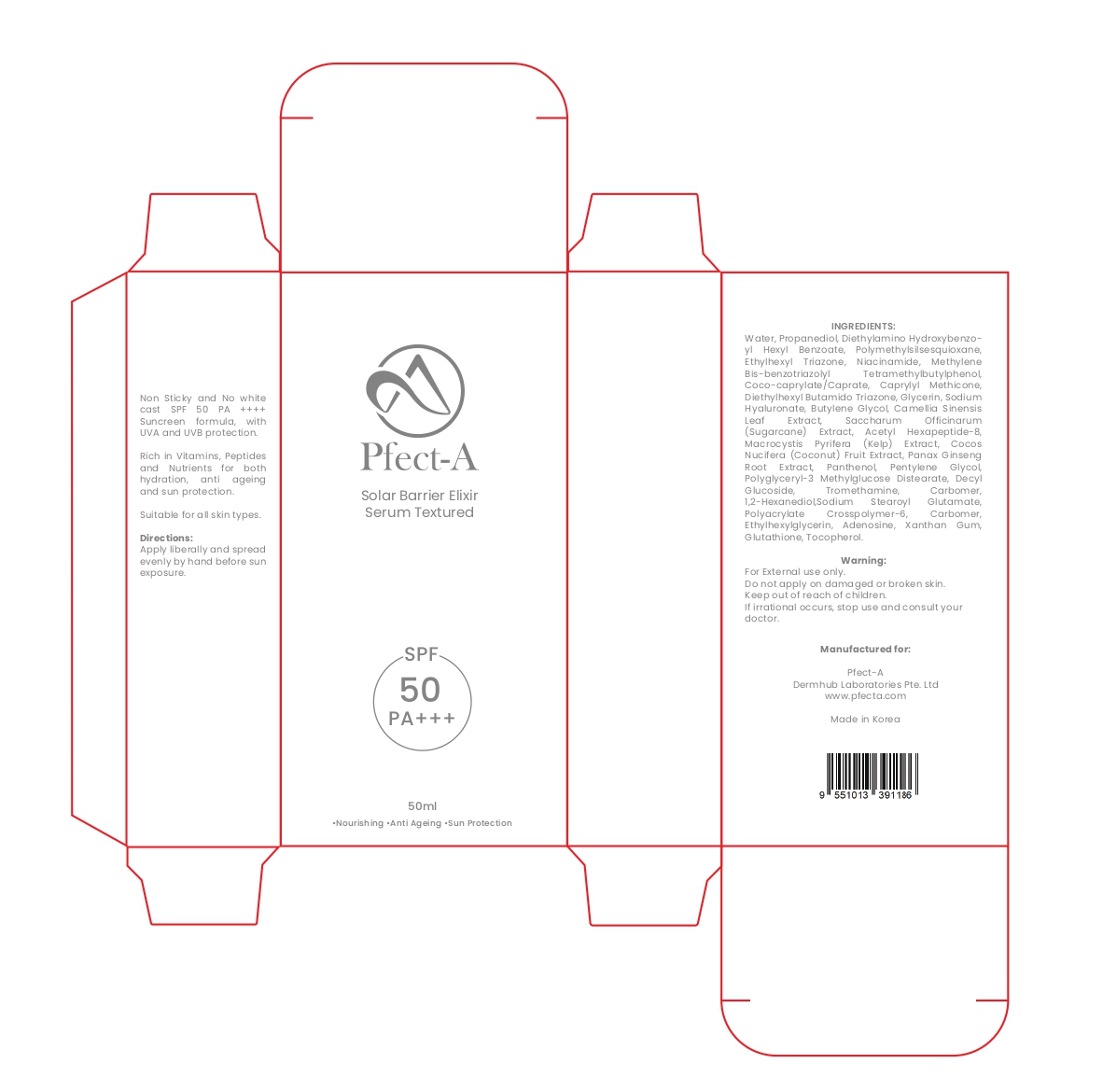 Pfect-A solar barrier Elixir Serum Textured
Pfect-A solar barrier Elixir Serum Textured