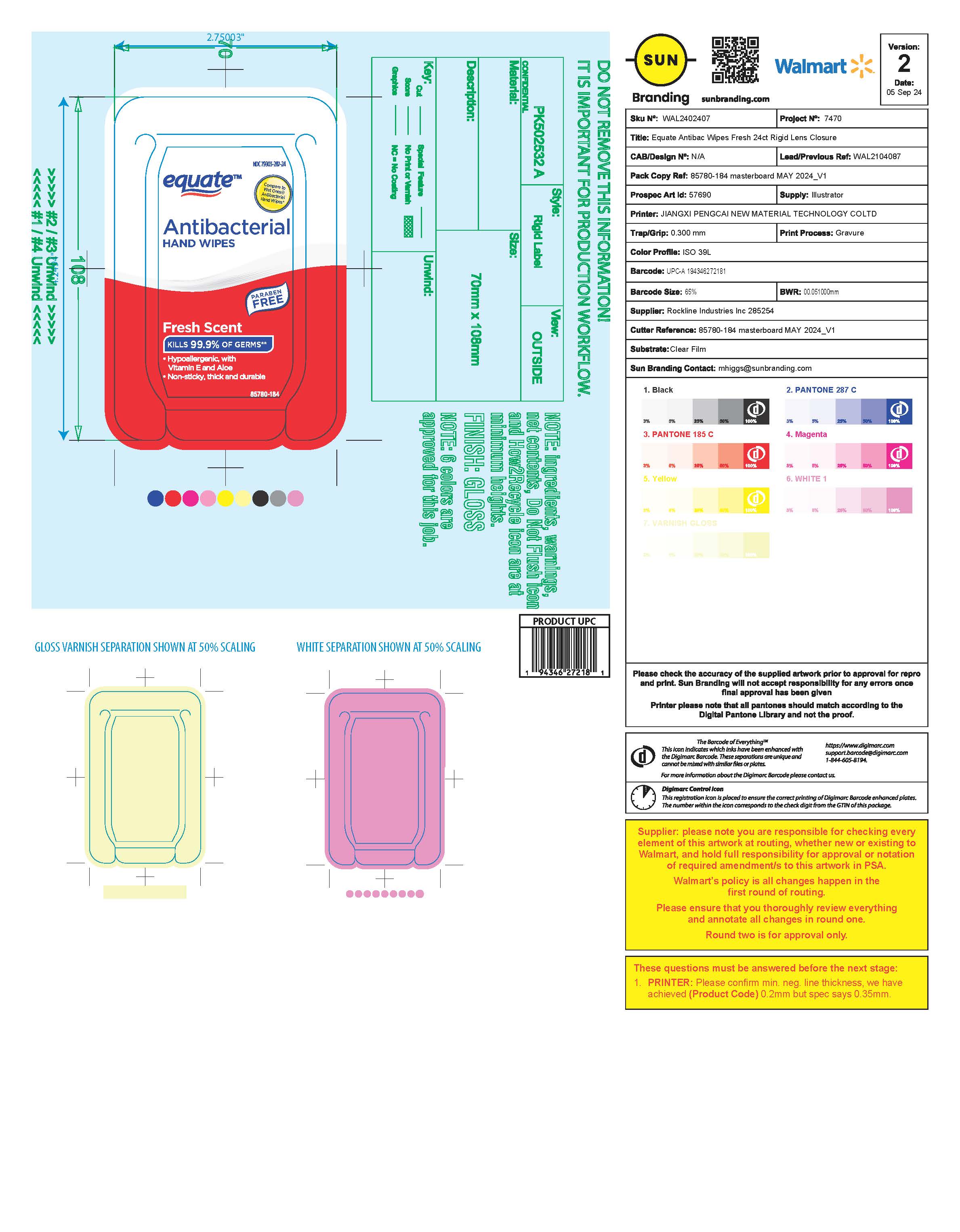
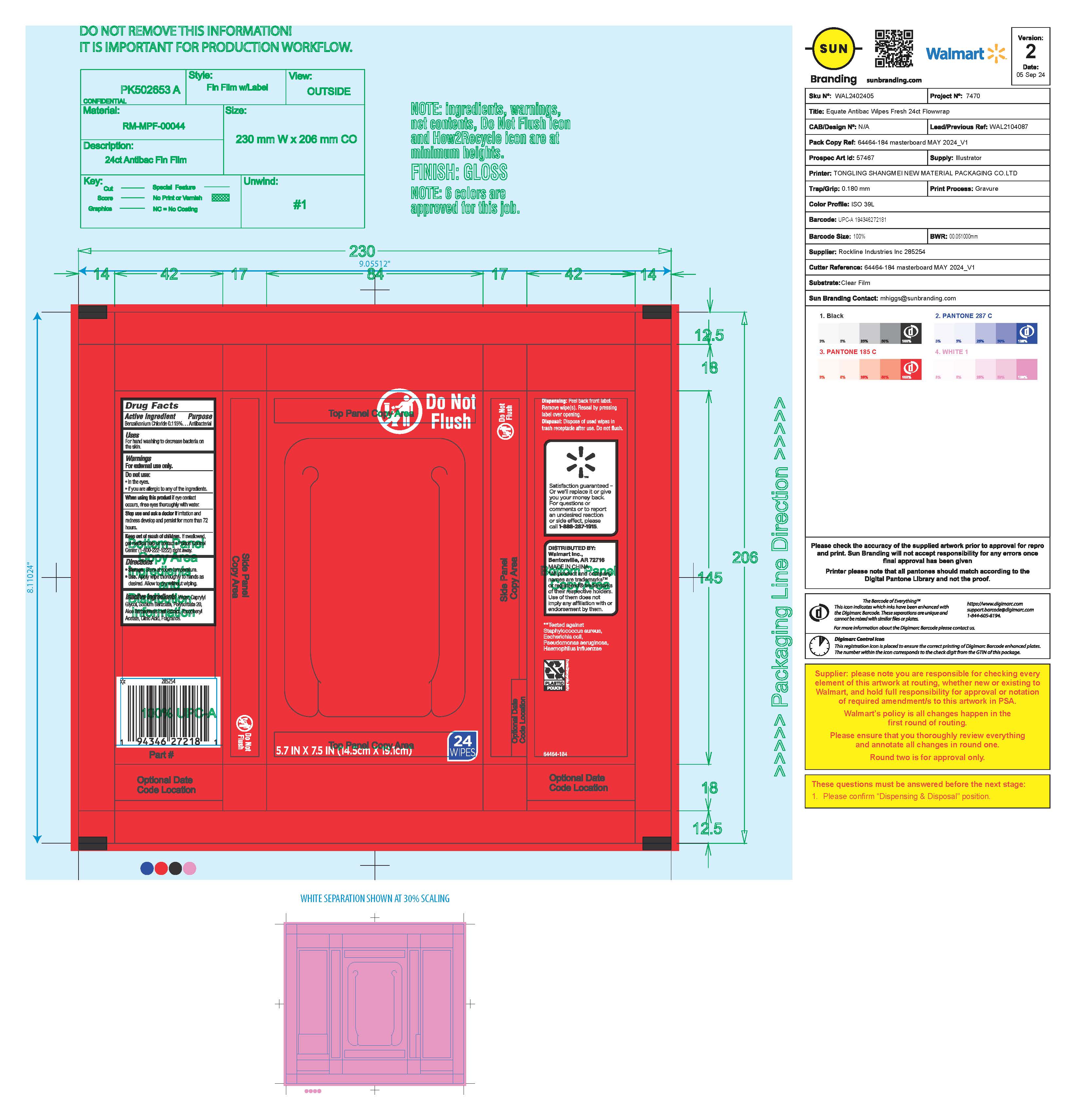
Label: EQUATE ANTIBACTERIAL WIPES FRESH- benzalkonium chloride cloth
- NDC Code(s): 79903-287-24
- Packager: Walmart, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children
- Directions
- Inactive ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
EQUATE ANTIBACTERIAL WIPES FRESH
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-287 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.115 g Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYSORBATE 20 (UNII: 7T1F30V5YH) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-287-24 24 in 1 PACKAGE; Type 0: Not a Combination Product 11/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 11/29/2024 Labeler - Walmart, Inc. (051957769)