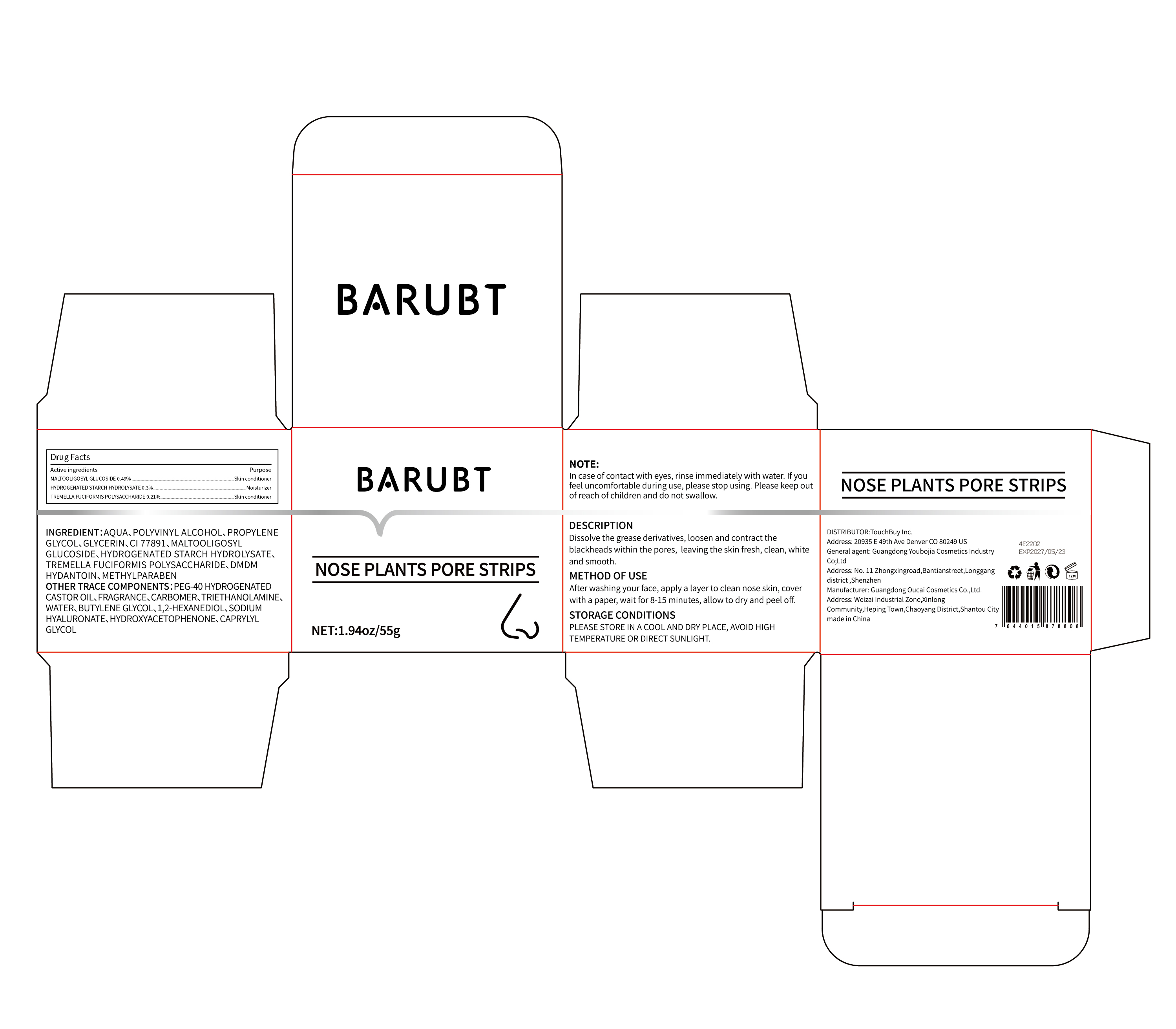
Label: BARUBT NOSE PLANTS PORE STRIPS- nose strips cream
- NDC Code(s): 84712-003-01
- Packager: Guangdong Youbaijia Cosmetic Industry Co., Ltd
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 8, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- WHEN USING
- QUESTIONS
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BARUBT NOSE PLANTS PORE STRIPS
nose strips creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84712-003 Route of Administration CUTANEOUS, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MALTOOLIGOSYL GLUCOSIDE (UNII: N91S91EFOG) (MALTOOLIGOSYL GLUCOSIDE - UNII:N91S91EFOG) MALTOOLIGOSYL GLUCOSIDE 0.27 g in 55 g HYDROGENATED STARCH HYDROLYSATE (UNII: 27F77DSJ5V) (HYDROGENATED STARCH HYDROLYSATE - UNII:27F77DSJ5V) HYDROGENATED STARCH HYDROLYSATE 0.165 g in 55 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 1.1 g in 55 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84712-003-01 55 g in 1 JAR; Type 0: Not a Combination Product 09/08/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/08/2024 Labeler - Guangdong Youbaijia Cosmetic Industry Co., Ltd (702314361) Establishment Name Address ID/FEI Business Operations Guangdong Youbaijia Cosmetic Industry Co., Ltd 702314361 label(84712-003) , manufacture(84712-003)