Label: BRONCHITOL- mannitol capsule
- NDC Code(s): 84639-212-01, 84639-212-04, 84639-212-14, 84639-212-56
- Packager: Arna Pharma Pty Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated September 3, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BRONCHITOL safely and effectively.
See full prescribing information for BRONCHITOL.
BRONCHITOL® (mannitol) inhalation powder, for oral inhalation use
Initial U.S. Approval: 1964INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- For Oral Inhalation Only. (2.1)
- The BRONCHITOL Tolerance Test is administered to identify patients who are appropriate for inhaled mannitol use. (2.1)
- Treatment of cystic fibrosis: 400 mg of BRONCHITOL (10 capsules) twice a day by oral inhalation, in the morning and evening, with the later dose taken 2-3 hours before bedtime. (2.2)
CONTRAINDICATIONS
- Hypersensitivity to mannitol or to any of the capsule components. (4)
- Failure to pass the BRONCHITOL Tolerance Test. (4)
WARNINGS AND PRECAUTIONS
- BRONCHITOL can cause bronchospasm, which can be severe in susceptible patients. Because of the risk of bronchospasm, prior to prescribing BRONCHITOL, perform the BRONCHITOL tolerance test (BTT). The BTT must be administered under the supervision of a healthcare practitioner who can treat severe bronchospasm. Do not prescribe BRONCHITOL if the patient fails the BTT. Patients who pass the BTT may experience bronchospasm with maintenance use of BRONCHITOL. Advise patients to premedicate with an inhaled short- acting bronchodilator prior to each administration of BRONCHITOL. If bronchospasm occurs, immediately discontinue BRONCHITOL. Treat bronchospasm with an inhaled short-acting bronchodilator. (2.1, 5.1)
- Hemoptysis can occur with BRONCHITOL use. Monitor patients with history of episodes of hemoptysis. If hemoptysis occurs, discontinue use of BRONCHITOL (5.2)
ADVERSE REACTIONS
Most common adverse reactions (≥3%) include cough, hemoptysis, oropharyngeal pain, vomiting, bacteria sputum identified, pyrexia, and arthralgia. (6.1) (6)
(6)
To report SUSPECTED ADVERSE REACTIONS, contact Pharmaxis Europe Limited at 1-888-276-0618 or FDA at 1-800-FDA-1088 or (6)
www.fda.gov/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION and (6)
FDA-approved patient labeling. (6)
Revised: 06/2024 (6)
See 17 for FDA-approved patient labeling.
Revised: 10/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Required Testing and Evaluation Prior to Prescribing BRONCHITOL (BRONCHITOL Tolerance Test)
2.2 Recommended Dosage for Treatment of Cystic Fibrosis
2.3 Use and Maintenance of Inhaler
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bronchospasm
5.2 Hemoptysis
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic and Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS & USAGE SECTION
-
2 DOSAGE AND ADMINISTRATION
2.1 Required Testing and Evaluation Prior to Prescribing BRONCHITOL (BRONCHITOL Tolerance Test)
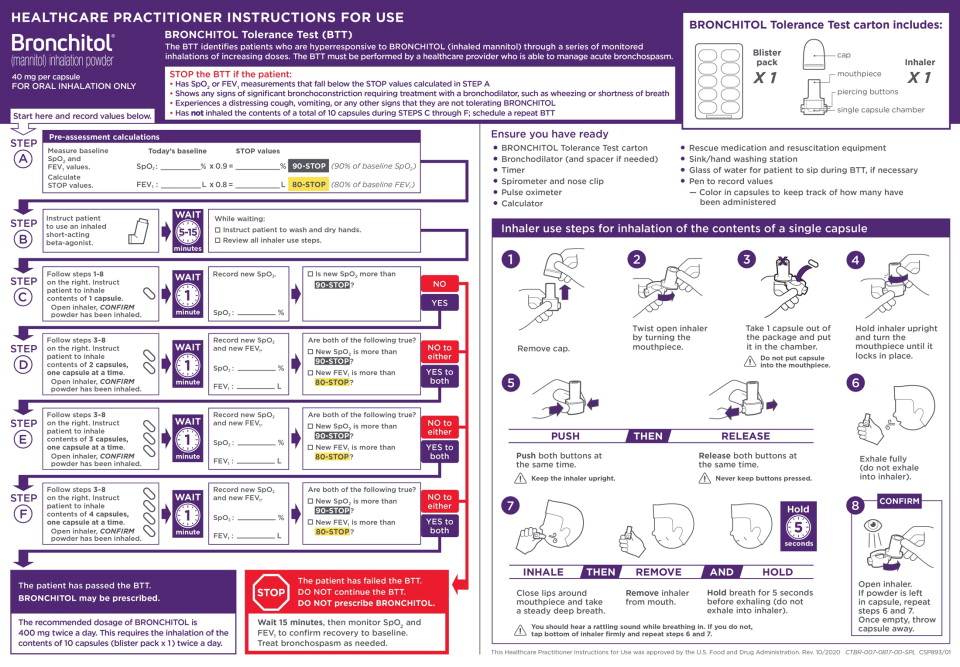
Prior to prescribing BRONCHITOL for treatment of cystic fibrosis, the BRONCHITOL Tolerance Test (BTT) must be administered and performed under the supervision of a healthcare practitioner who is able to manage acute bronchospasm, to identify patients who are suitable candidates for BRONCHITOL maintenance therapy.
- Perform BTT to identify patients who experience bronchospasm, a decrease in FEV1, or a decrease in oxygen saturation with administration of BRONCHITOL. If a patient experiences any of these events during the BTT, the patient has failed the BTT. Do not prescribe BRONCHITOL. If a patient does not experience any of these events during BTT, the patient has passed the BTT and is a candidate for BRONCHITOL therapy.
- Ensure that rescue medication and resuscitation equipment are available for immediate use during the BTT.
- Do not perform the BTT if the patient is considered clinically unstable.
See the BTT Healthcare Practitioner (HCP) Instructions for Use (IFU) for complete instructions and to avoid medication errors
associated with BTT dosing and procedures.
Do not use BRONCHITOL add-on maintenance therapy in patients who fail the BTT [see Contraindications (4)].2.2 Recommended Dosage for Treatment of Cystic Fibrosis
For patients who have passed the BTT, the recommended dosage of BRONCHITOL is 400 mg twice a day by oral inhalation (the contents of 10 capsules administered individually) via the inhaler [see Dosage and Administration (2.1)].
A short-acting bronchodilator should be administered by oral inhalation, 5-15 minutes before every dose of BRONCHITOL.
BRONCHITOL should be taken once in the morning and once in the evening, with the later dose taken at least 2-3 hours before
bedtime.2.3 Use and Maintenance of Inhaler
Instruct patients on safe hygiene practices (clean and dry hands thoroughly) and correct inhaler use, including loading of capsules and proper inhalation technique per the Patient Instructions for Use.
The BRONCHITOL inhaler should be discarded and replaced after 7 days of use. If the inhaler does need to be washed, the patient should allow the inhaler to thoroughly air dry before next use. - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Bronchospasm
BRONCHITOL Tolerance Test
BRONCHITOL can cause bronchospasm, which can be severe in susceptible individuals. Because of the risk of bronchospasm, prior to prescribing BRONCHITOL, perform the BRONCHITOL Tolerance Test (BTT), to identify patients who are appropriate for maintenance treatment with BRONCHITOL. The BTT must be administered under the supervision of a healthcare practitioner who can treat severe bronchospasm. In clinical trials, 896 adult patients with cystic fibrosis underwent the BTT and 72 patients (8%) failed or did not complete the BTT. Do not prescribe BRONCHITOL if the patient fails the BTT.Maintenance Therapy
Bronchospasm may occur during inhalation of BRONCHITOL, even in patients who have passed the BTT. An inhaled short-acting bronchodilator must be administered 5-15 minutes before administration of each dose during maintenance therapy. In clinical studies, bronchospasm or bronchial hyperreactivity was reported in 4 of 414 adult patients (1.0%) receiving BRONCHITOL as maintenance therapy and in 2 of 347 adult patients (0.6%) receiving control (50 mg inhaled mannitol), even though these patients had passed the BTT. If bronchospasm occurs following dosing of BRONCHITOL, it should immediately be discontinued and treated with an inhaled short-acting bronchodilator or as medically appropriate.5.2 Hemoptysis
Hemoptysis may occur with BRONCHITOL use. Hemoptysis was reported in 43 (10.4%) adult patients receiving BRONCHITOL and in 33 (9.5%) adult patients receiving control (50 mg inhaled mannitol) during clinical studies. In patients aged 6 years to 17 years, hemoptysis was reported in 12 of 154 (7.8%) patients who received BRONCHITOL and in 2 of 105 (1.9%) patients who received control. BRONCHITOL has not been studied in patients with a history of episodes of significant hemoptysis (volume greater than 60 mL) in the previous 3 months. BRONCHITOL should be discontinued in the event of hemoptysis. BRONCHITOL is not indicated for use in children and adolescents.
- 6 ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of BRONCHITOL in pregnant women. The available data on BRONCHITOL use in pregnant women are not sufficient to inform any drug-associated risks for major birth defects and miscarriage. Based on animal reproduction studies, no evidence of structural alterations was observed when mannitol was administered to pregnant rats and mice during organogenesis at doses up to approximately 20 and 10 times, respectively, the maximum recommended daily inhalation dose (MRDID) in humans [ see Data]. There are risks to the mother associated with cystic fibrosis in pregnancy [see Clinial Considerations]. BRONCHITOL should be used during pregnancy only if the potential benefit justifies the potential risk to the mother and fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Cystic fibrosis may increase the risk for preterm delivery. Data
Animal Data
In animal reproduction studies, oral administration of mannitol to pregnant rats and mice during the period of organogenesis did not cause fetal structural alterations. The mannitol dose in rats and mice was approximately 20 and 10 times the maximum recommended human daily inhalation dose (MRDID) in humans, respectively, (on a mg/m 2 basis at maternal doses of 1600 mg/kg/day in both species).
8.2 Lactation
Risk Summary
It is not known whether BRONCHITOL is excreted in human breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BRONCHITOL and any potential adverse effects on the breastfed child from BRONCHITOL or from the underlying maternal condition.
8.4 Pediatric Use
BRONCHITOL is not indicated for use in children and adolescents. The safety and effectivenss of BRONCHITOL have not been established in pediatric patients for cystic fibrosis. Patients aged 6 years to 17 years were included in two 26- week, double-blind clinical trials (Trials 2 and 3). In these trials, 154 patients under 18 years of age received BRONCHITOL and 105 patients received control (50 mg inhaled mannitol). Hemoptysis was reported in 12 of 154 (7.8%) patients who received BRONCHITOL and in 2 of 105 (1.9%) patients who received control.
8.5 Geriatric Use
Clinical trials of BRONCHITOL did not include sufficient numbers of patients with cystic fibrosis who were 65 years of age and older to allow evaluation of safety and efficacy in this population.
8.6 Hepatic and Renal Impairment
Clinical trials of BRONCHITOL did not include patients with hepatic or renal impairment. No specific dose recommendations for these patient populations are available. However, an increase in systemic exposure of mannitol can be expected in patients with renal impairment based on the kidney being its primary route of elimination.
- 10 OVERDOSAGE
-
11 DESCRIPTION
BRONCHITOL (mannitol) inhalation powder contains D-Mannitol (referred to throughout as mannitol) as the active ingredient. Mannitol is a hexahydric sugar alcohol, with the following chemical name hexane-1,2,3,4,5,6-hexol and chemical structure:
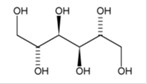
Mannitol is a white or almost white crystalline powder or free-flowing granules with an empirical formula of C6H14O6 and molecular weight of 182.2. Mannitol is freely soluble in water, and very slightly soluble in alcohol. Mannitol shows polymorphism.
BRONCHITOL contains mannitol powder spray dried into particles of respirable size filled into clear, colorless hard gelatin capsules. There are no inactive ingredients in BRONCHITOL.
The accompanying white plastic inhaler is comprised of a mouthpiece, blue piercing buttons, capsule chamber, and a removable cap. A blister pack consists of 10 capsules, each containing 40 mg mannitol. After a capsule is placed in the
capsule chamber and pierced by firmly pressing and releasing the buttons on the side of the device, the powder within the capsule becomes exposed and ready for dispersion into the airstream generated by the patient upon inhalation through the mouthpiece. Under standardized in vitro test conditions, the inhaler delivers 32.2 mg of mannitol per inhalation when tested at a flow rate of 60 L/min for 2 seconds. The actual amount of drug delivered to the lungs will depend on patient factors, such as inspiratory flow profile.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism of action of BRONCHITOL in improving pulmonary function in cystic fibrosis patients is unknown.
12.3 Pharmacokinetics
Absorption
Following oral inhalation of 635 mg, the mean mannitol peak plasma concentration (Cmax) was 13.71 mcg/mL while the mean extent of systemic exposure (AUC) was 73.15 mcg•hr/mL. The mean time to peak plasma concentration (Tmax) after oral inhalation was 1.5 hour.
Distribution
Based on intravenous administration, the volume of distribution of mannitol was 34.3 L.
Elimination
Metabolism
Mannitol is metabolized in a CYP-independent manner through the glycolytic pathway via dehydrogenation to fructose. The extent of metabolism of mannitol appears to be small. This is evident from a urinary excretion of about 87% of unchanged drug after an intravenous dose to healthy patients.
Excretion
Following oral inhalation, the elimination half-life of mannitol was 4.7 hours. The mean terminal elimination half-life for mannitol in plasma remained unchanged regardless of the route of administration (oral, inhalation, and intravenous). The urinary excretion rate versus time profile for mannitol was consistent for all routes of administration. The total clearance after intravenous administration was 5.1 L/hr while the renal clearance was 4.4 L/hr. Therefore, the clearance of mannitol was predominately via the kidney. Following inhalation of 635 mg of mannitol in 18 healthy patients, about 55% of the total dose was excreted in the urine as unchanged mannitol. Following oral or intravenous administration of a 500 mg dose, the corresponding values were 54% and 87% of the dose, respectively.
Specific Populations
Patients with Hepatic and Renal Impairment: Formal pharmacokinetic studies using BRONCHITOL have not been conducted in patients with hepatic or renal impairment. Since the drug is eliminated primarily via the kidney, an increase in systemic exposure can be expected in renally impaired patients.
Drug Interaction Studies
No formal drug interaction studies have been conducted with BRONCHITOL.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In 2-year carcinogenicity studies in rats and mice mannitol did not show evidence of carcinogenicity at oral dietary concentrations up to 5% (or 7,500 mg/kg on a mg/kg basis). These doses were approximately 55 and 30 times the
MRHDID, respectively, on a mg/m 2 basis.
Mutagenesis
Mannitol tested negative in the following assays: bacterial gene mutation assay, in vitro mouse lymphoma assay, in vitro chromosomal aberration assay in WI-38 human cells, in vivo chromosomal aberration assay in rat bone marrow, in vivo dominant lethal assay in rats, and in vivo mouse micronucleus assay.
Impairment of Fertility
The effect of inhaled mannitol on fertility has not been investigated.
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
BRONCHITOL (mannitol) inhalation powder:
- 40 mg of mannitol per capsule
- capsules are clear, colorless and imprinted in black with “PXS” on cap and “40 mg” on body
- supplied in cartons containing 10, 140 or 560 capsules in blister packs co-packaged with 1, 1, and 4 inhalers respectively in a carton
BRONCHITOL is provided in 3 commercial presentations:
Pack Quantities
Inhalers
Capsules
NDC Number
4-week Treatment Pack (4 x 7-day treatment packs)
4
560
84639-212-56
7-day Treatment Pack
1
140
84639-212-14
Bronchitol Tolerance Test
1
10
84639-212-04
BRONCHITOL should be stored between 68°F-77°F (20°C-25°C) with excursions permitted between 59°F-86°F (15°C- 30°C). [See USP Controlled Room Temperature]. Do not refrigerate. Do not freeze.
BRONCHITOL should only be used with the provided inhaler, which is a white plastic inhaler comprised of a mouthpiece, blue piercing buttons, capsule chamber, and a removable cap. All remaining unused (opened and unopened) blister packs and the inhalers should be properly discarded. Be sure to read the accompanying BRONCHITOL instructions completely before administration. If you have any questions, contact the supplier at 1-888-276-2144.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Patient Instructions for Use).
BRONCHITOL Tolerance Test
Inform patients that a BRONCHITOL Tolerance Test is required prior to beginning treatment with BRONCHITOL. The
BRONCHITOL Tolerance Test must be performed by a healthcare practitioner equipped to monitor oxygen saturation (SpO 2), perform spirometry (FEV 1), and manage acute bronchospasm.
Inhaled Short-Acting Bronchodilator Use
Instruct patients that an inhaled short-acting bronchodilator such as albuterol must always be administered 5 to 15 minutes prior to every dose of BRONCHITOL.
Bronchospasm
Prior to administration, inform patients that bronchospasm can occur with inhalation of BRONCHITOL. If patient experiences bronchospasm, instruct the patient to discontinue BRONCHITOL and contact their healthcare practitioner right away.
Hemoptysis
Inform patients that hemoptysis can occur with inhalation of BRONCHITOL. If a patient experiences hemoptysis, instruct patients to discontinue BRONCHITOL and contact their healthcare practitioner right away.
Administration
Instruct patients on the proper administration of BRONCHITOL with the inhaler. The recommended dosage is 10 capsules (400 mg) twice a day. This requires inhaling the contents of 10 capsules administered individually once in the morning and once at least 2-3 hours before bed.
Manufactured by:
Arna Pharma Pty Ltd.
20 Rodborough Rd Frenchs Forest NSW 2086 AUSTRALIA
Manufactured for:
Pharmaxis Europe Limited
108 Q House Furze Road,
Sandyford Dublin 18,
D18AY29 Ireland
BRONCHITOL® is a registered trademark of Pharmaxis Europe Limited.
- HEALTHCARE PRACTITIONER INSTRUCTIONS FOR USE
-
PATIENT INSTRUCTIONS FOR USE
BRONCHITOL® (mannitol) inhalation powder for oral inhalation use
This Patient Instructions for Use contains information on how to take BRONCHITOL.
Each BRONCHITOL box contains:
7-day Treatment Pack
4-week Treatment Pack
- 140 Capsules (14 blister packs)
- 560 Capsules (56 blister packs)
- 1 Inhaler
- 4 Inhalers
- Prescribing Information
- Prescribing Information

Important Information You Need to Know Before Using BRONCHITOL
- Do not use BRONCHITOL until your healthcare provider has performed the BRONCHITOL Tolerance Test (BTT) and approved you for treatment. This is to make sure you get the right treatment if you have a severe reaction.
- For oral inhalation only
- Do not swallow BRONCHITOL capsules.
- Use an inhaled short-acting bronchodilator 5 to15 minutes before every dose of BRONCHITOL.
- Use BRONCHITOL 2 times each day. Inhale through your mouth (oral inhalation) the capsule contents in 10 single BRONCHITOL capsules:
- 1 time in the morning
- 1 time at least 2 to 3 hours before bedtime
Preparing to use BRONCHITOL
BRONCHITOL is supplied to people in cartons containing 140 or 560 capsules in blister packs. Supplies you will need to use BRONCHITOL:
- 1 Blister pack
- 1 Inhaler
- Bronchodilator (and spacer for bronchodilator if needed)
- Sink or hand washing station
Before using BRONCHITOL:
Use an inhaled bronchodilator 5 to15 minutes before using BRONCHITOL (See Figure A).
Clean and dry hands well (See Figure B).
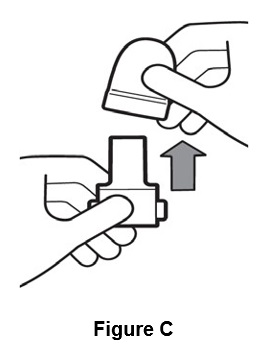
Inhaler use steps for inhalation of the contents of a single capsule: Step 1. Remove cap (See Figure C).
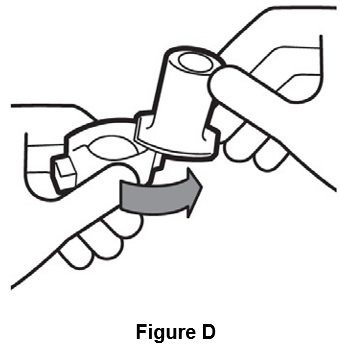
Step 2. Twist open inhaler by turning the mouthpiece to the right. (See Figure D).
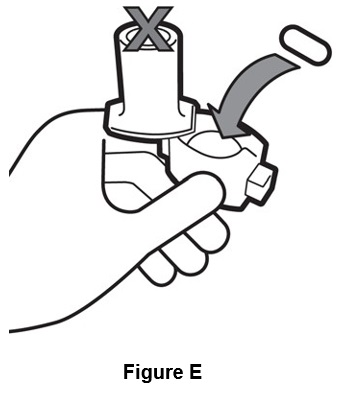
Step 3. Take 1 capsule out of the blister pack and put it in the chamber. (See Figure E).
Do not place capsule into the mouthpiece of the inhaler.
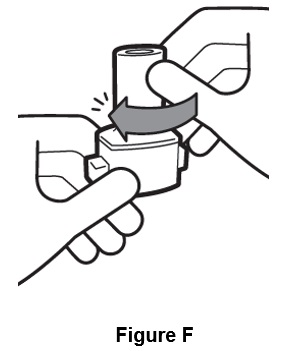
Step 4. Hold inhaler upright and turn the mouthpiece to the left until it locks in place. (See Figure F).
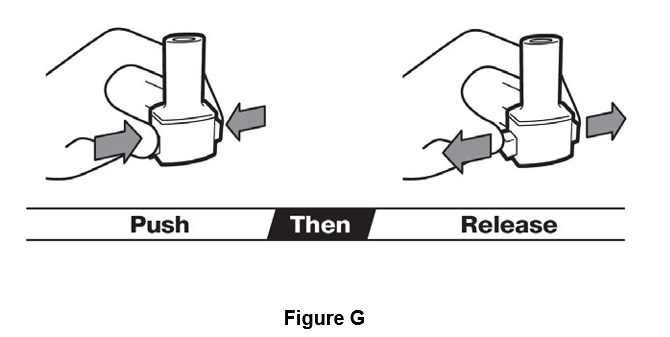
Step 5. Push both piercing buttons at the same time. Release both piercing buttons at the same time (See Figure G).
Keep inhaler upright. Never keep piercing buttons pressed.
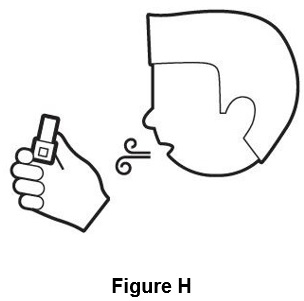
Step 6. Breathe out (exhale) fully (See Figure H).
Do not breathe out into inhaler.
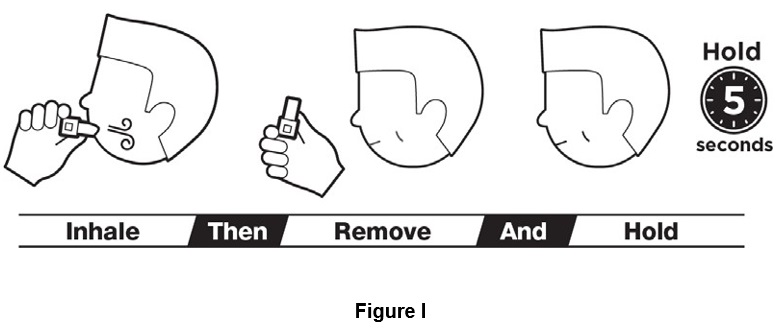
Step 7. Close lips around the mouthpiece and take a steady deep breath in through your mouth. Do not breathe through your nose. Remove inhaler from mouth. Hold breath for 5 seconds before exhaling, do not breathe out (exhale) into inhaler (See Figure I).
You should hear a rattling sound while breathing in. If you do not, tap bottom of inhaler firmly and repeat steps 6 and 7.
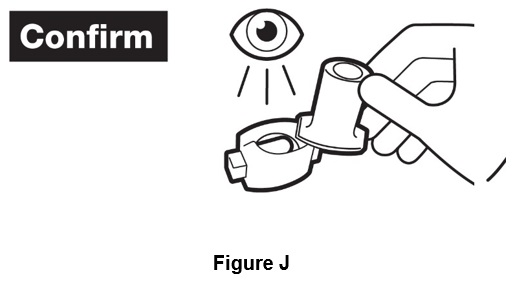
Step 8. Open the inhaler by turning the cap to the right. If powder is left in capsule, repeat steps 6 and 7. After the capsule is empty, throw away. (See Figure J).
Step 9. Repeat steps 3 to 8 for all 10 capsules in 1 blister pack (See Figure K).
Breathe in (inhale) contents of each capsule one after another until all 10 capsules in the blister pack are used.
Step 10. After inhaling contents of all 10 capsules, close mouthpiece and place cap on inhaler (See Figure L).
Step 11. Continue using BRONCHITOL for 7 days then throw away (discard) the inhaler (See Figure M).
How should I store BRONCHITOL?
- Store BRONCHITOL at room temperature between 68°F to77°F (20°C to 25°C).
- If your BRONCHITOL capsules are stored at temperatures more than 86°F (30°C), throw them away.
- Do not freeze BRONCHITOL.
- Do not refrigerate BRONCHITOL.
- Keep BRONCHITOL and all medicines out of the reach of children.
Cleaning your BRONCHITOL inhaler.
- Your inhaler should give you the correct dose of medicine for 7 days without needing cleaning. However, if your inhaler does need cleaning:
- Make sure your inhaler is empty.
- Wash your inhaler in warm water with the mouthpiece open.
- Shake it until there are no large water droplets left in the inhaler.
- Leave it to dry in the air by laying it on its side with the mouthpiece open.
- Allow the inhaler to dry fully (or completely) after it has been washed.
Caring for your BRONCHITOL inhaler.
- Keep your inhaler dry and always make sure your hands are completely dry before using it.
- Do not breathe or cough into your inhaler.
- Do not take your inhaler apart.
- Do not place a capsule directly into the mouthpiece of your inhaler.
- Do not leave a used capsule in your inhaler chamber.
- Use a new inhaler after 7 days.
- If your inhaler breaks, call to your healthcare provider.
For more information about BRONCHITOL or how to use your inhaler, call 1-888-276-2144.
Manufactured by:
Arna Pharma Pty Ltd.
20 Rodborough Road
Frenchs Forest NSW
2086 AUSTRALIA
Manufactured for:
Pharmaxis Europe Limited
108 Q House Furze Road,
Sandyford Dublin 18,
D18AY29 Ireland
1-888-276-2144
BRONCHITOL is registered in the US Patent and Trademark Office.
This Patient Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 06/2024
-
Principle Display Panel - 40 mg 4 Week Carton
Principal Display Panel - 40 mg 4 Week Carton Label
Rx Only
NDC 84639-212-56
Caution: Federal Law Prohibits Dispensing Without A Prescription
Bronchitol®
(mannitol) inhalation powder
40 mg per capsule
FOR ORAL INHALATION ONLY
Do Not Swallow Bronchitol Capsules
Complete entire package (56 Blister Packs) for 28 days treatment
Contents:
56 Blister Cards: 560 capsules (40 mg each)
4 Bronchitol devices: For use with enclosed capsules only
See package insert for complete dosage information
For more information, call 1-888-661-9260.

-
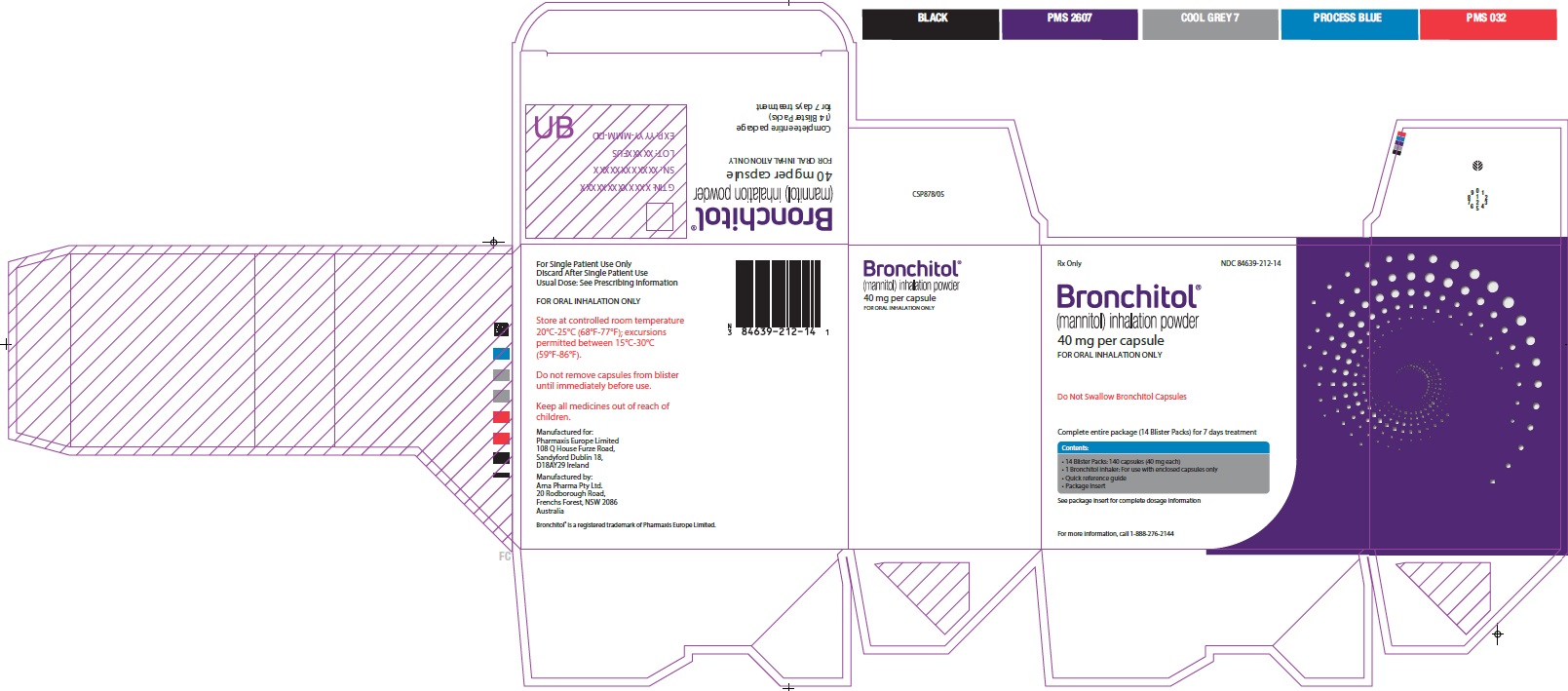
Principal Display Panel - 40 mg 7 Day Carton Label
Principal Display Panel - 40 mg 7 Day Carton Label
Rx Only
NDC 84639-212-14
Bronchitol®
(mannitol) inhalation powder
40 mg per capsule
FOR ORAL INHALATION ONLY
Do Not Swallow Bronchitol Capsules
Complete entire package (14 Blister Packs) for 7 days treatment
Contents:
- 14 Blister Packs: 140 capsules (40 mg each)
- 1 Bronchitol inhaler: For use with enclosed capsules only
- Quick reference guide
- Package Insert
See package insert for complete dosage information
For more information, call 1-888-276-0618.
-
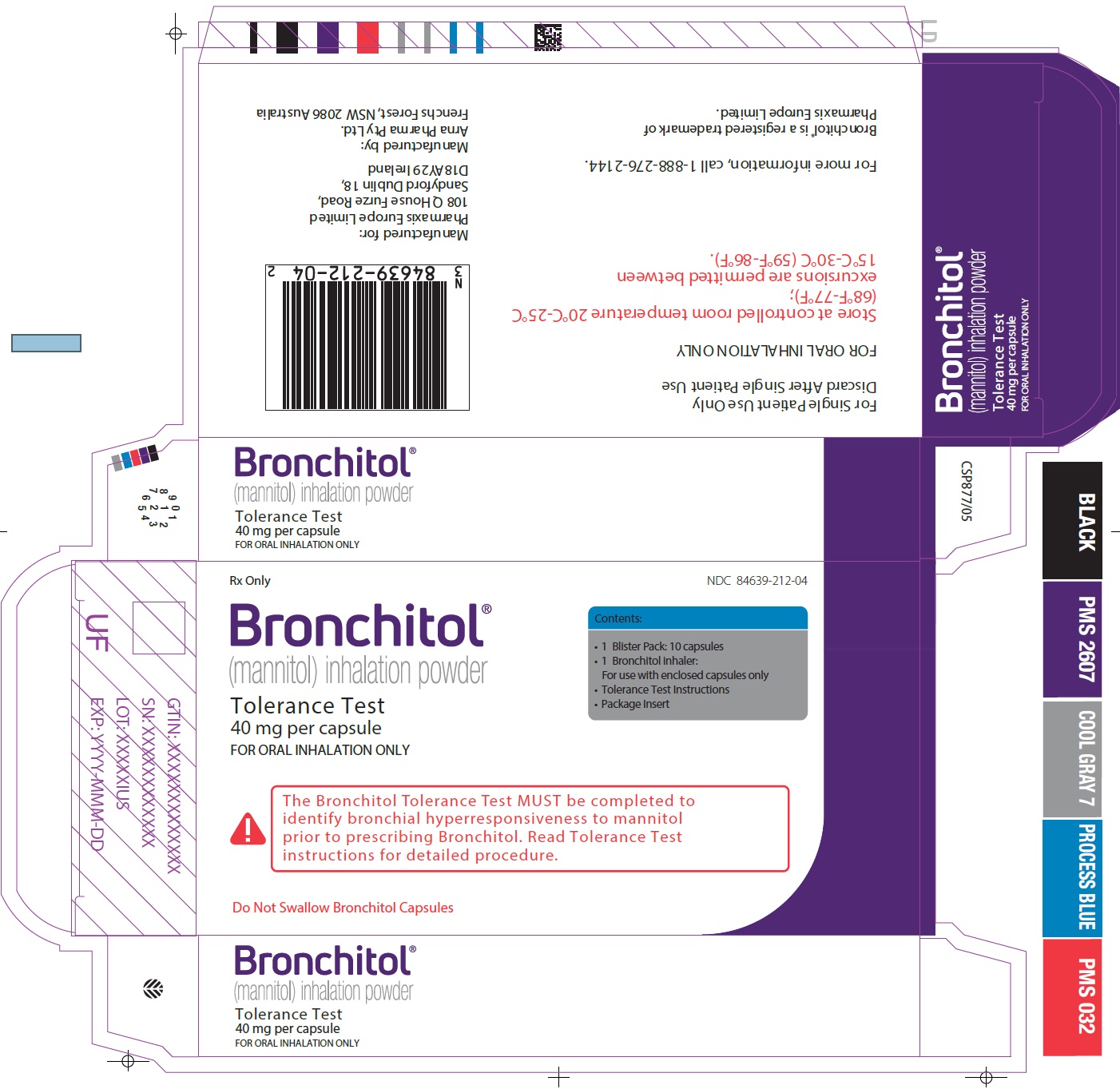
Principal Display Panel - 40 mg Tolerance Test Carton Label
Principal Display Panel - 40 mg Tolerance Test Carton Label
Rx Only
NDC 84639-212-04
Bronchitol®
(mannitol) inhalation powder
Tolerance Test
40 mg per capsule
FOR ORAL INHALATION ONLY
Contents:
- 1 Blister Pack: 10 capsules
- 1 Bronchitol inhaler:
For use with enclosed capsules only
- Tolerance Test Instructions
- Package Insert
The Bronchitol Tolerance Test MUST be completed to
identify bronchial hyperresponsiveness to mannitol
prior to prescribing Bronchitol. Read Tolerance Test
instructions for detailed procedure.Do Not Swallow Bronchitol Capsules
-
INGREDIENTS AND APPEARANCE
BRONCHITOL
mannitol capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:84639-212 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANNITOL (UNII: 3OWL53L36A) (MANNITOL - UNII:3OWL53L36A) MANNITOL 40 mg in 10 Product Characteristics Color white (Clear capsule with white powder) Score no score Shape CAPSULE (CAPSULE) Size 15mm Flavor Imprint Code PXS;40;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84639-212-04 1 in 1 CARTON 10/02/2024 1 NDC:84639-212-01 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:84639-212-56 4 in 1 BOX 10/02/2024 2 NDC:84639-212-14 14 in 1 CARTON 2 NDC:84639-212-01 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:84639-212-14 14 in 1 CARTON 10/02/2024 3 NDC:84639-212-01 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202049 10/02/2024 Labeler - Arna Pharma Pty Ltd (751073905)