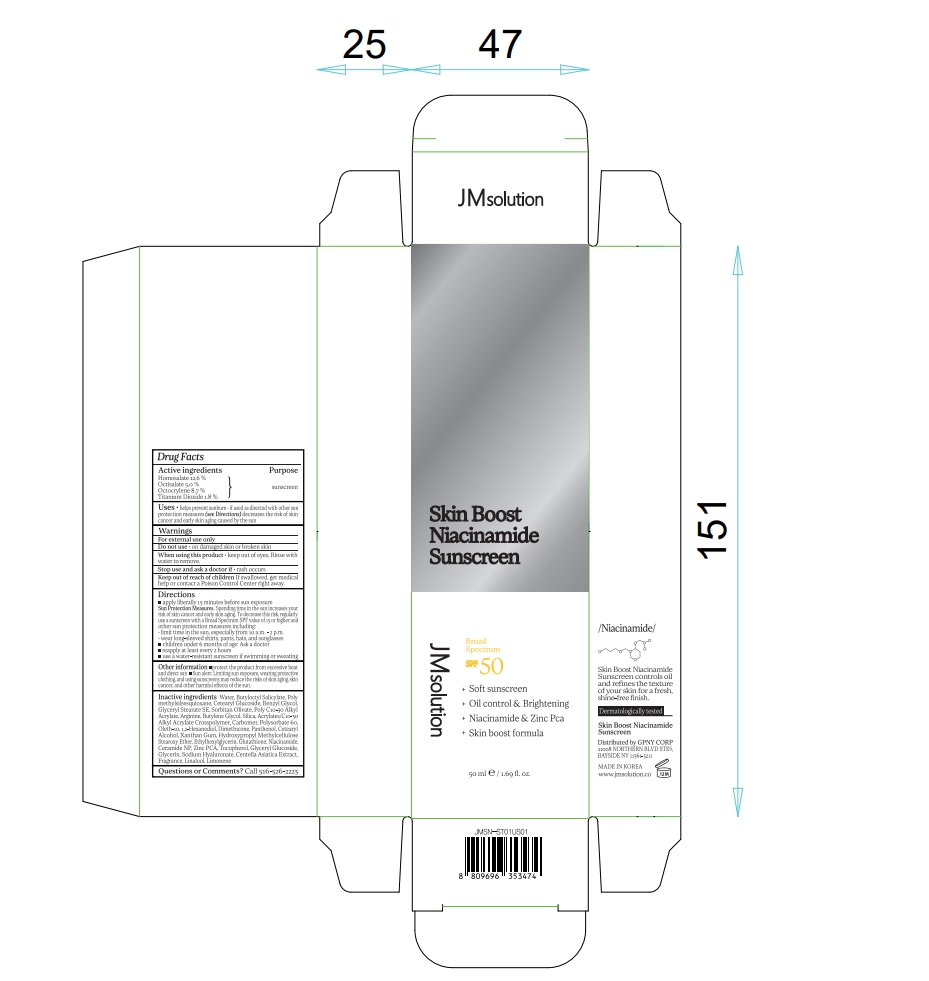
Label: JMSOLUTION SKIN BOOST NIACINAMIDE SUNSCREEN- homosalate, octisalate, octocrylene, titanium dioxide cream
- NDC Code(s): 84754-051-02
- Packager: GPNY CORP
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
■ apply liberally 15 minutes before sun exposure
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. - 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
■ children under 6 months of age: Ask a doctor
■ reapply at least every 2 hours
■ use a water-resistant sunscreen if swimming or sweating - Other information
-
Inactive ingredients
Water, Butyloctyl Salicylate, Polymethylsilsesquioxane, Cetearyl Glucoside, Benzyl Glycol, Glyceryl Stearate SE, Sorbitan Olivate, Poly C10-30 Alkyl Acrylate, Arginine, Butylene Glycol, Silica, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Carbomer, Polysorbate 60, Oleth-10, 1,2-Hexanediol, Dimethicone, Panthenol, Cetearyl Alcohol, Xanthan Gum, Hydroxypropyl Methylcellulose Stearoxy Ether, Ethylhexylglycerin, Glutathione, Niacinamide, Ceramide NP, Zinc PCA, Tocopherol, Glyceryl Glucoside, Glycerin, Sodium Hyaluronate, Centella Asiatica Extract, Fragrance, Linalool, Limonene
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JMSOLUTION SKIN BOOST NIACINAMIDE SUNSCREEN
homosalate, octisalate, octocrylene, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84754-051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4.35 g in 50 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 2.5 g in 50 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.9 g in 50 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 6.3 g in 50 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) XANTHAN GUM (UNII: TTV12P4NEE) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PANTHENOL (UNII: WV9CM0O67Z) ETHYLENE GLYCOL MONOBENZYL ETHER (UNII: 06S8147L47) CERAMIDE NP (UNII: 4370DF050B) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) DIMETHICONE (UNII: 92RU3N3Y1O) TOCOPHEROL (UNII: R0ZB2556P8) NIACINAMIDE (UNII: 25X51I8RD4) WATER (UNII: 059QF0KO0R) SORBITAN OLIVATE (UNII: MDL271E3GR) POLYSORBATE 60 (UNII: CAL22UVI4M) OLETH-10 (UNII: JD797EF70J) GLUTATHIONE (UNII: GAN16C9B8O) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) ARGININE (UNII: 94ZLA3W45F) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) ZINC PIDOLATE (UNII: C32PQ86DH4) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LIMONENE, (+)- (UNII: GFD7C86Q1W) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84754-051-02 1 in 1 BOX 11/07/2024 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/07/2024 Labeler - GPNY CORP (108497367) Establishment Name Address ID/FEI Business Operations Rebom Co., Ltd. 695951708 manufacture(84754-051)