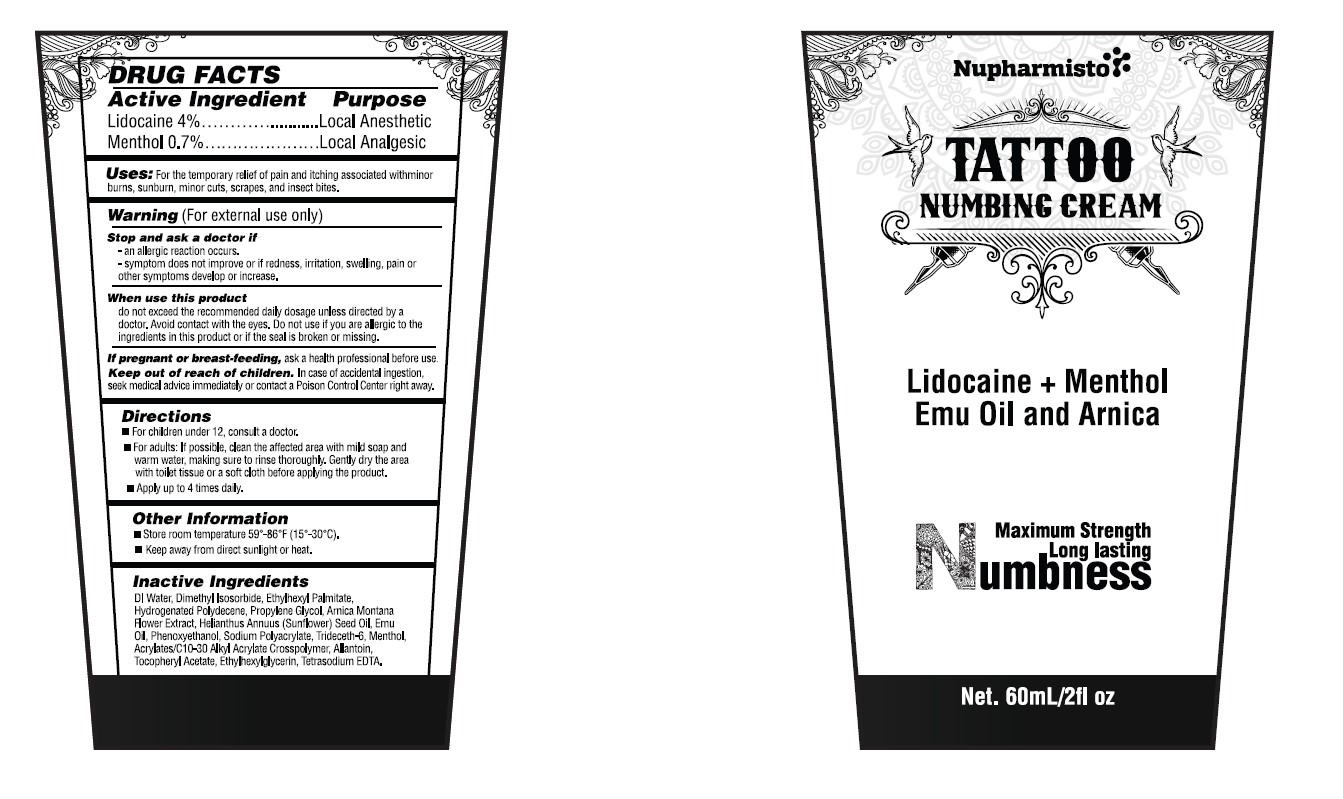
Label: NUPHARMISTO TATTOO NUMBING CREAM 60ML- lidocaine cream
- NDC Code(s): 71331-119-02
- Packager: Orange Lab, Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- If pregnant of breast feeding section
- other information (storage)
- Inactive Ingredients
- Directions for use
- Questions ? Contact us.
- dosage and how to use it correctly section
- Indications and usage section
- Label Images
-
INGREDIENTS AND APPEARANCE
NUPHARMISTO TATTOO NUMBING CREAM 60ML
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71331-119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.7 g in 100 g Inactive Ingredients Ingredient Name Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) ALLANTOIN (UNII: 344S277G0Z) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EMU OIL (UNII: 344821WD61) TRIDECETH-6 (UNII: 3T5PCR2H0C) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) HYDROGENATED POLYDECENE (1500 CST) (UNII: 4YI0729529) SUNFLOWER OIL UNSAPONIFIABLES (UNII: T7ZE2WA4MB) ETHYLHEXYL PALMITATE (UNII: 2865993309) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) EDETATE SODIUM (UNII: MP1J8420LU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71331-119-02 1 in 1 BOX 09/01/2024 1 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/01/2024 Labeler - Orange Lab, Inc (004862271)