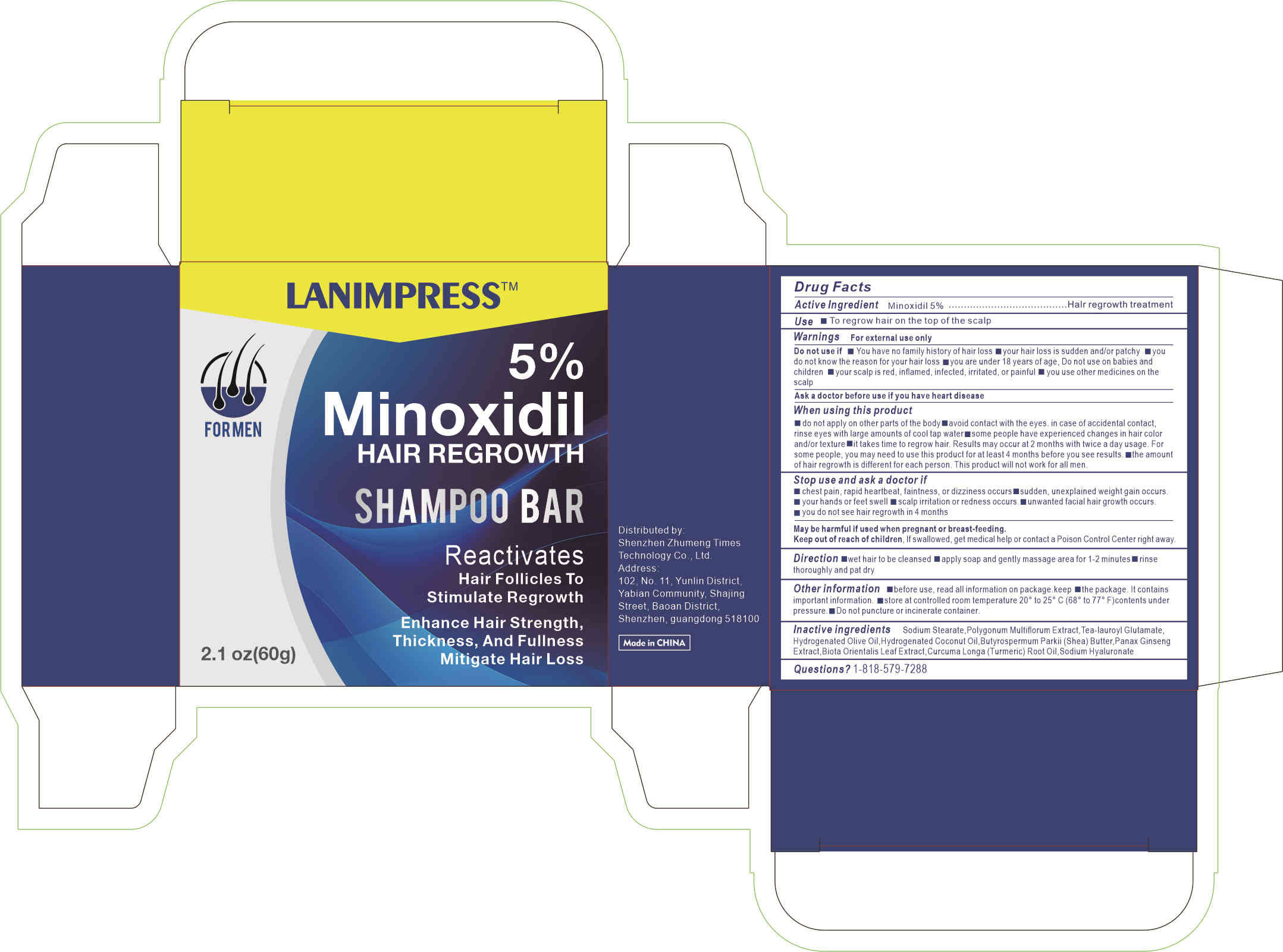
Label: 5%MINOXIDIL HAIR REGROWTH liquid
- NDC Code(s): 84372-007-01
- Packager: Shenzhen Zhumeng Times Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 20, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Do not use if
You have no famlly history of hair loss
your hair loss is sudden and/or patchy
you do not know the reason for your hair loss
you are under 18 years of age, Do not use on babies and children
your scalp is red, inflamed, infected, irritated, or painful
you use other medicines on the scalp
Ask a doctor before use if you have heart disease
- Dosage and administration
-
Do not use
Stop use and ask a doctor if
chest pain, rapid heartbeat, faintness, or dizziness occurs
sudden, unexplained weight gain occurs
your hands or feet swell
scalp irritation or redness occurs
unwanted facial hair growth occurs
you do not see hair regrowth in 4 months
May be harmful if used when pregnant or breast-feeding.
Keep out of reach of children, lf swallowed, get medical help or contact a Poison Control Center right away.
-
When using section
do not apply on other parts of the body
avoid contact with the eyes. in case of accidental contact, rinse eyes with large amounts of cool tap water
some people have experienced changes in hair color and/or texture
it takes time to regrow hair. Results may occur at 2 months with twice a day usage. For some people, you may need to use this product for at least 4 months before you see results.
the amount of hair regrowth is different for each person. This product will notwork for all men.
- stop use
- Keep out of reach of children.
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
5%MINOXIDIL HAIR REGROWTH
5%minoxidil hair regrowth liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84372-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 g Inactive Ingredients Ingredient Name Strength TRIETHANOLAMINE LAUROYL GLUTAMATE (UNII: 0X7WD3EN1I) PLATYCLADUS ORIENTALIS LEAF (UNII: 32E5V7G32B) SODIUM STEARATE (UNII: QU7E2XA9TG) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) HYALURONATE SODIUM (UNII: YSE9PPT4TH) HYDROGENATED OLIVE OIL (UNII: 53839415GI) ASIAN GINSENG (UNII: CUQ3A77YXI) TURMERIC OIL (UNII: 6KGS8SP16U) REYNOUTRIA MULTIFLORA ROOT (UNII: AUZ3VD75MC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84372-007-01 60 g in 1 BOX; Type 0: Not a Combination Product 08/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/19/2024 Labeler - Shenzhen Zhumeng Times Technology Co., Ltd. (631852731) Establishment Name Address ID/FEI Business Operations Shenzhen Zhumeng Times Technology Co., Ltd. 631852731 manufacture(84372-007)