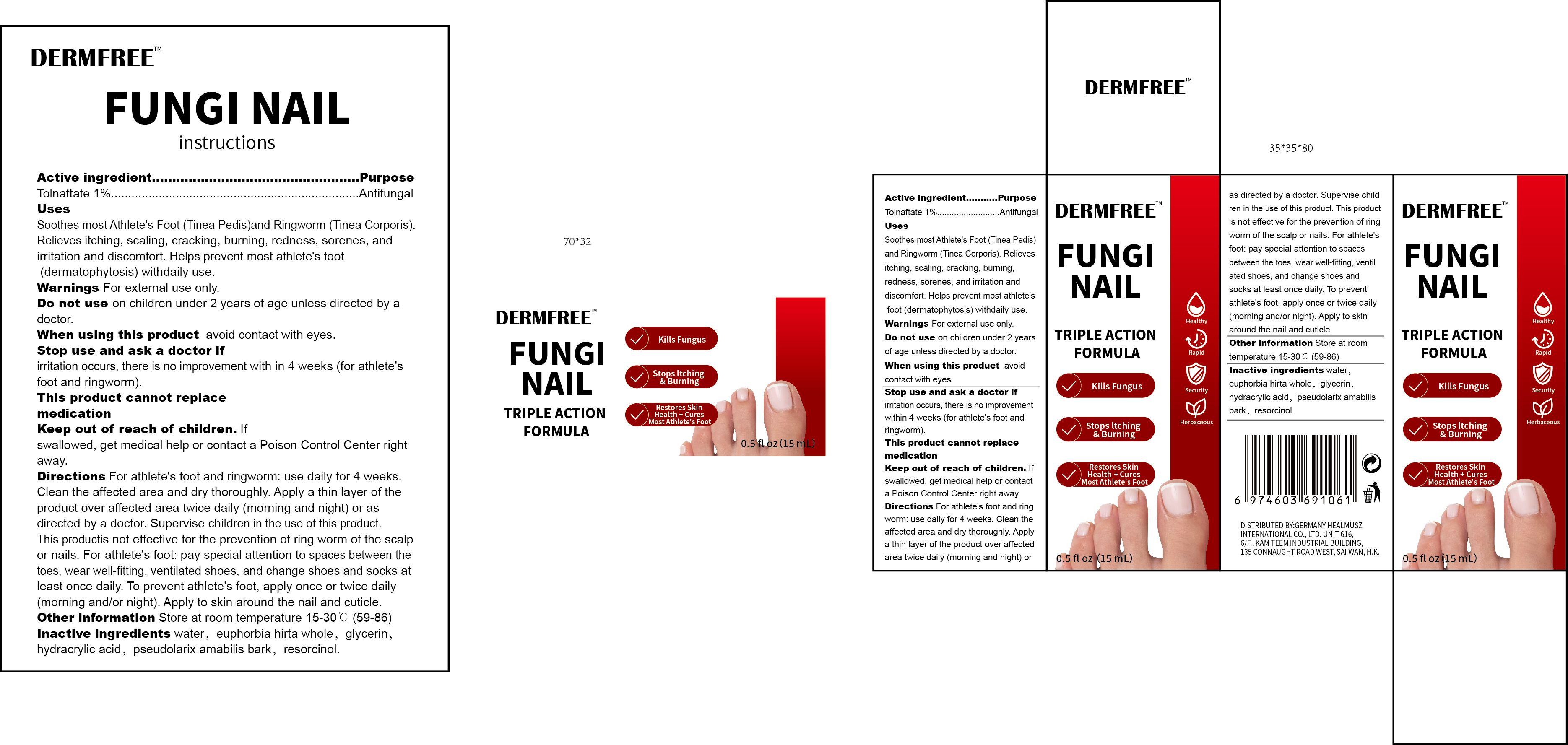
Label: DERMFREE FUNGI NAIL- tolnaftate 1% fungi nail liquid
- NDC Code(s): 84010-026-01
- Packager: Jiangxi Hemei Pharmaceutical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
-
Directions
For athlete's foot and ringworm: use daily for 4 weeks. Clean the affected area and dry thoroughly. Apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor. Supervise children in the use of this product.This productis not effective for the prevention of ring worm of the scalp or nails. For athlete's foot: pay special attention to spaces between the toes, wear well-fitting, ventilated shoes, and change shoes and socks at least once daily. To prevent athlete's foot, apply once or twice daily(morning and/or night).Apply to skin around the nail and cuticle.
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMFREE FUNGI NAIL
tolnaftate 1% fungi nail liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84010-026 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength HYDRACRYLIC ACID (UNII: C4ZF6XLD2X) PSEUDOLARIX AMABILIS BARK (UNII: 49G13A93VE) EUPHORBIA HIRTA WHOLE (UNII: L13YF113GN) RESORCINOL (UNII: YUL4LO94HK) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84010-026-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 08/18/2024 Labeler - Jiangxi Hemei Pharmaceutical Co., Ltd (724892056) Establishment Name Address ID/FEI Business Operations Jiangxi Hemei Pharmaceutical Co., Ltd 724892056 manufacture(84010-026)