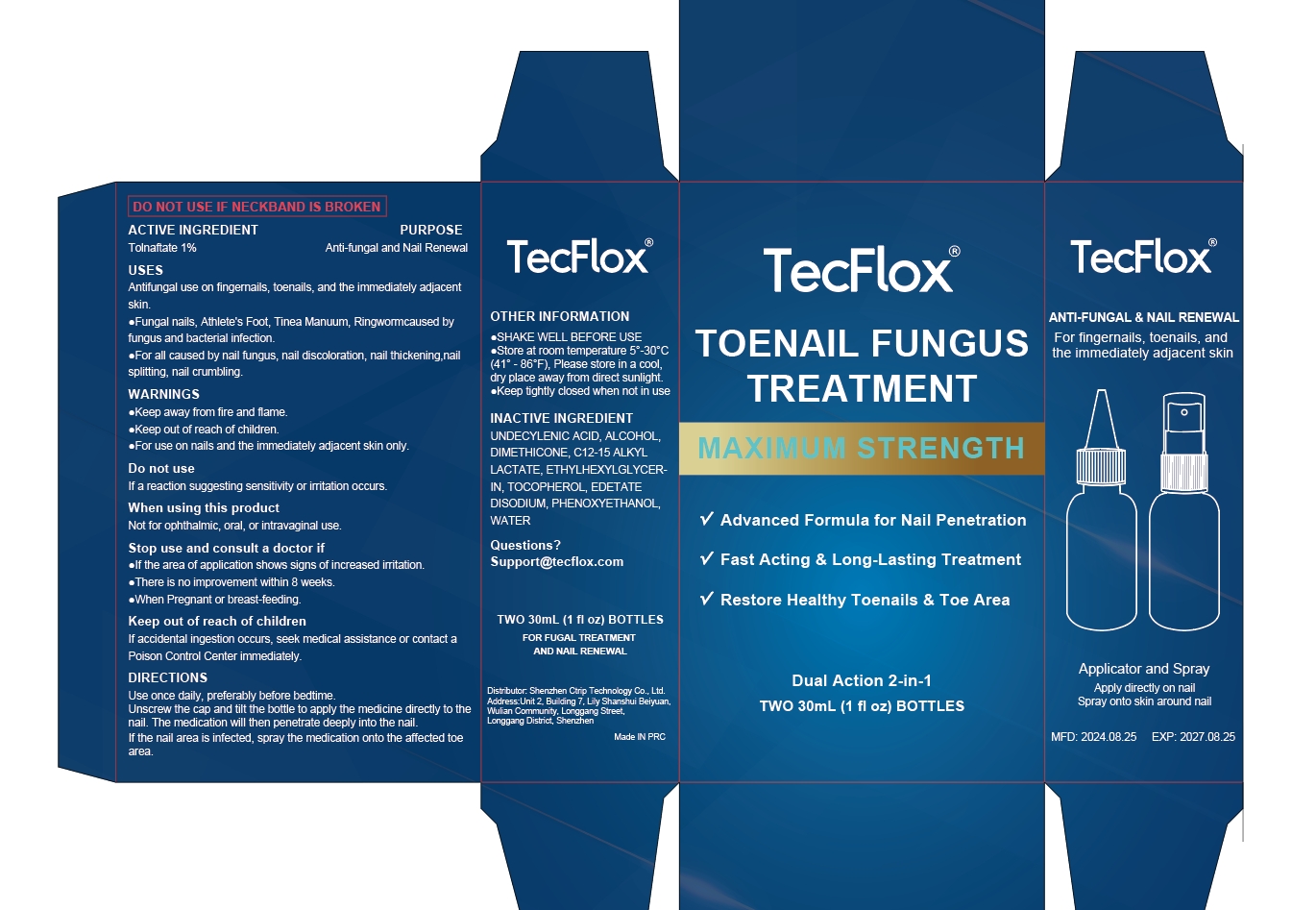
Label: TOENAIL FUNGUS TREATMENT MAXIMUM STRENGTH LIQUID- tolnaftate liquid
- NDC Code(s): 84614-002-01
- Packager: Shenzhen Ctrip Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOENAIL FUNGUS TREATMENT MAXIMUM STRENGTH LIQUID
tolnaftate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84614-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM (UNII: 7FLD91C86K) DIMETHICONE (UNII: 92RU3N3Y1O) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84614-002-01 2 in 1 PACKAGE 08/23/2024 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 08/23/2024 Labeler - Shenzhen Ctrip Technology Co., Ltd. (403113060) Establishment Name Address ID/FEI Business Operations Shenzhen Ctrip Technology Co., Ltd. 403113060 manufacture(84614-002)