Label: QUALITY CHOICE LICE TREATMENT- piperonyl butoxide, pyrethrum extract kit
- NDC Code(s): 83324-268-01
- Packager: Chain Drug Marketing Association
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 9, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Uses
- Purpose
-
Warnings
For external use only
Do not use
- near eyes
- inside nose, mouth, or vagina
- on lice in eyebrows or eyelashes. See a doctor if lice are present in these areas.
Ask a doctor before use if you are
- allergic to ragweed. May cause breathing difficulty or an asthmatic attack.
When using this product
- keep eyes tightly closed and protect eyes with a washcloth or towel
- if product gets into eyes, flush with water right away
- scalp itching or redness may occur
-
Directions
- Important: Read warnings before use
- Adults and children 2 years and over:
Inspect
- check each household member with a magnifying glass in bright light for lice/nits (eggs)
- look for tiny nits near scalp, beginning at back of neck and behind ears
- examine small sections of hair at a time
- unlike dandruff which moves when touched, nits stick to the hair
- if either lice or nits are found, treat with this product
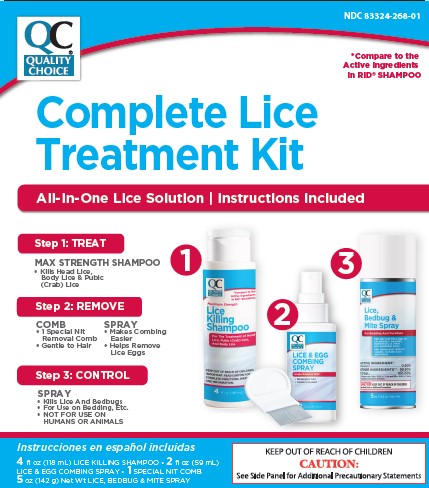
Treat
- apply thoroughly to DRY HAIR or other affected area. For head lice, first apply behind ears and to back of neck.
- allow product to remain for 10 minutes, but no longer
- use warm water to form a lather, shampoo, then thoroughly rinse
- for head lice, towel dry hair and comb out tangles
Remove lice and their eggs (nits)
- use a fine-tooth or special lice/nit comb. Remove any remaining nits by hand (using a throw-away glove).
- hair should remain slightly damp while removing nits
- if hair dries during combing, dampen slightly with water
- for head lice, part hair into sections. Do one section at a time starting on top of the head. Longer hair may take 1 to 2 hours.
- lift a 1-to 2-inch wide strand of hair. Place comb as close to scalp as possible and comb with a firm, even motion away from scalp.
- pin back each strand of hair after combing
- clean comb often. Wipe nits away with tissue and discard in a plastic bag. Seal bag and discard to prevent lice from coming back.
- after combing, thoroughly recheck for lice/nits. Repeat combing if necessary.
- check daily for any lice/nits that you missed
- a second treatment must be done in 7 to 10 days to kill any newly hatched lice
- if infestation continues, see a doctor for other treatments
- Children under 2 years: ask a doctor
- Other information
- Inactive ingredients
- Consumer Information - Package Insert
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
QUALITY CHOICE LICE TREATMENT
piperonyl butoxide, pyrethrum extract kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83324-268 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83324-268-01 1 in 1 CARTON; Type 0: Not a Combination Product 09/09/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 118 mL Part 1 of 1 LICE KILLING
piperonyl butoxide, pyrethrum extract shampooProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIPERONYL BUTOXIDE (UNII: LWK91TU9AH) (PIPERONYL BUTOXIDE - UNII:LWK91TU9AH) PIPERONYL BUTOXIDE 40 mg in 1 mL PYRETHRUM EXTRACT (UNII: ZUM06L90GV) (PYRETHRUM EXTRACT - UNII:ZUM06L90GV) PYRETHRUM EXTRACT 3.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYQUATERNIUM-10 (400 MPA.S AT 2%) (UNII: HB1401PQFS) AMMONIUM LAURETH-2 SULFATE (UNII: 698O4Z48G6) ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) PEG-25 HYDROGENATED CASTOR OIL (UNII: 0ZNO9PJJ9J) Product Characteristics Color yellow (cloudy to transparent, cream to straw) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M031 02/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M031 09/09/2024 Labeler - Chain Drug Marketing Association (011920774) Registrant - Weeks & Leo Co., Inc. (005290028) Establishment Name Address ID/FEI Business Operations Weeks & Leo Co., Inc. 005290028 manufacture(83324-268)



 Quality Choice
Quality Choice