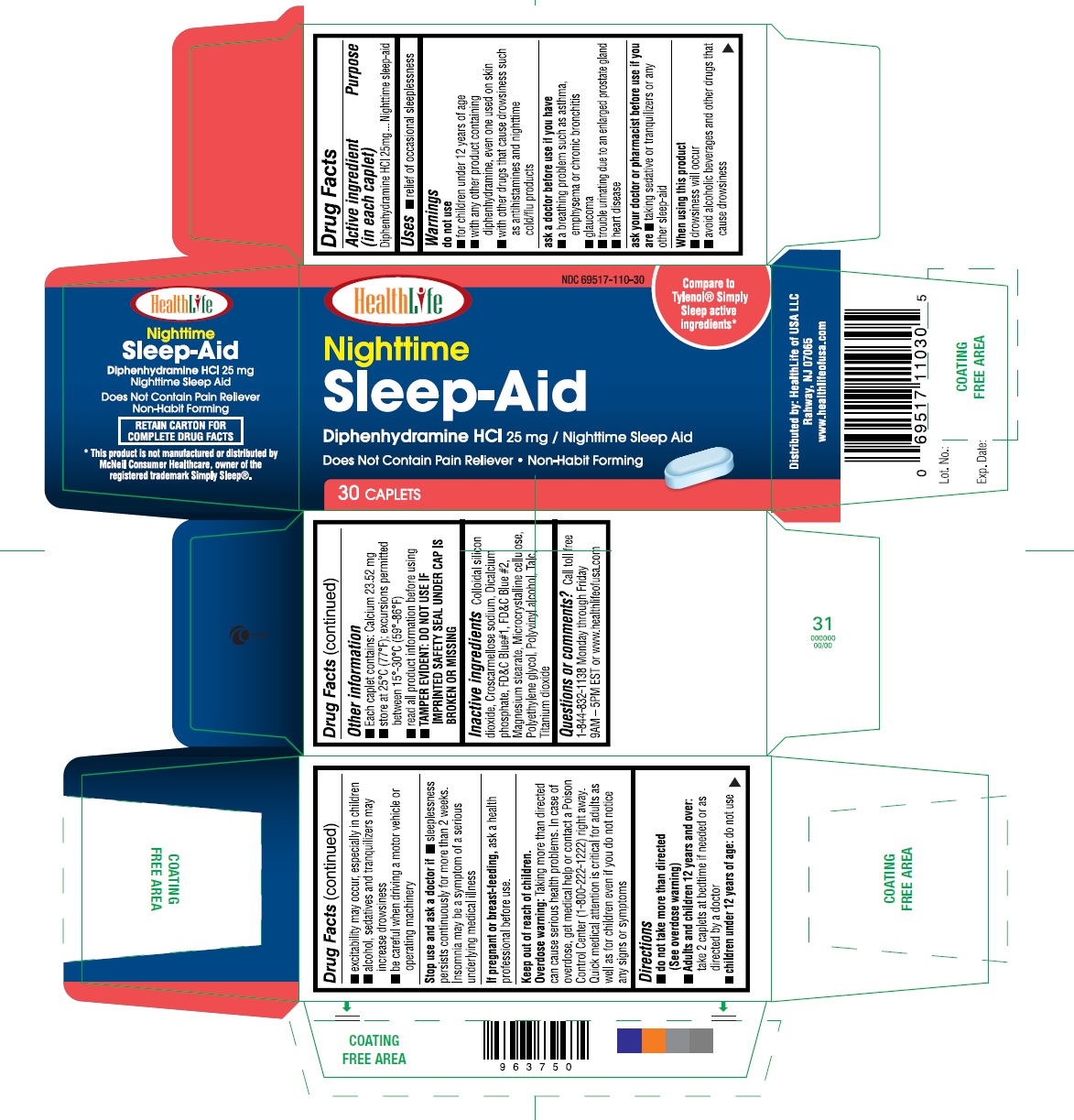
Label: NIGHTTIME SLEEP AID- diphenhydramine hcl 25 mg tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 69517-110-01, 69517-110-02, 69517-110-05, 69517-110-25, view more69517-110-30, 69517-110-50 - Packager: HealthLife of USA
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 5, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other drugs that cause drowsiness such as antihistamines and nighttime cold/flu products
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
-
heart disease
Ask a doctor or pharmacist before use if you are
taking sedative or tranquilizers or any other sleep-aid
When using this product
- drowsiness will occur
- avoid alcoholic beverages and other drugs that cause drowsiness
- excitability may occur, especially in children
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
Keep out of reach of children.
Overdose warning: Taking more than directed can cause serious health problems. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
- Directions
- Other Information
- Inactive Ingredients
-
Questions or comments?
Call toll free 1-844-832-1138 Monday through Friday 9AM – 5PM EST or www.healthlifeofusa.com
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NIGHTTIME SLEEP AID
diphenhydramine hcl 25 mg tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69517-110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYVINYL ALCOHOL (UNII: 532B59J990) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color blue Score no score Shape CAPSULE Size 11mm Flavor Imprint Code EC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69517-110-25 25 in 1 BOX 06/01/2017 1 2 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:69517-110-50 50 in 1 BOX 06/01/2017 2 2 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:69517-110-02 2 in 1 POUCH 06/01/2017 3 2 in 1 POUCH; Type 0: Not a Combination Product 4 NDC:69517-110-01 1000 in 1 BOTTLE 06/01/2017 4 NDC:69517-110-05 500 in 1 BOTTLE 4 NDC:69517-110-30 30 in 1 BOTTLE 4 1 in 1 CARTON; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/01/2017 Labeler - HealthLife of USA (079656178) Establishment Name Address ID/FEI Business Operations Elysium Pharmaceutical Ltd. 915664486 manufacture(69517-110)