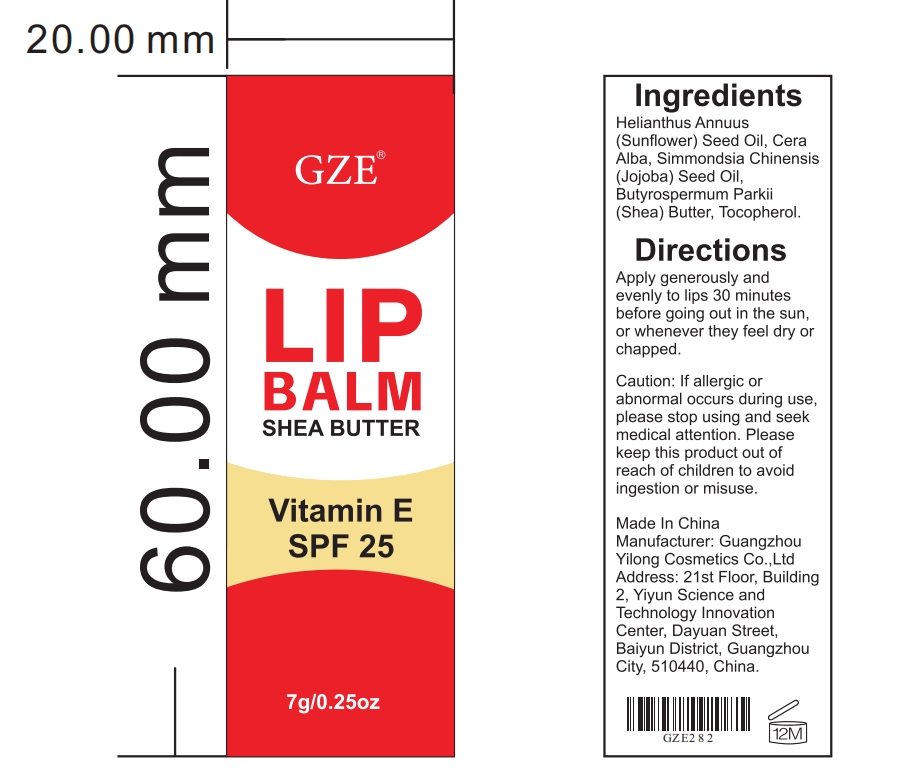
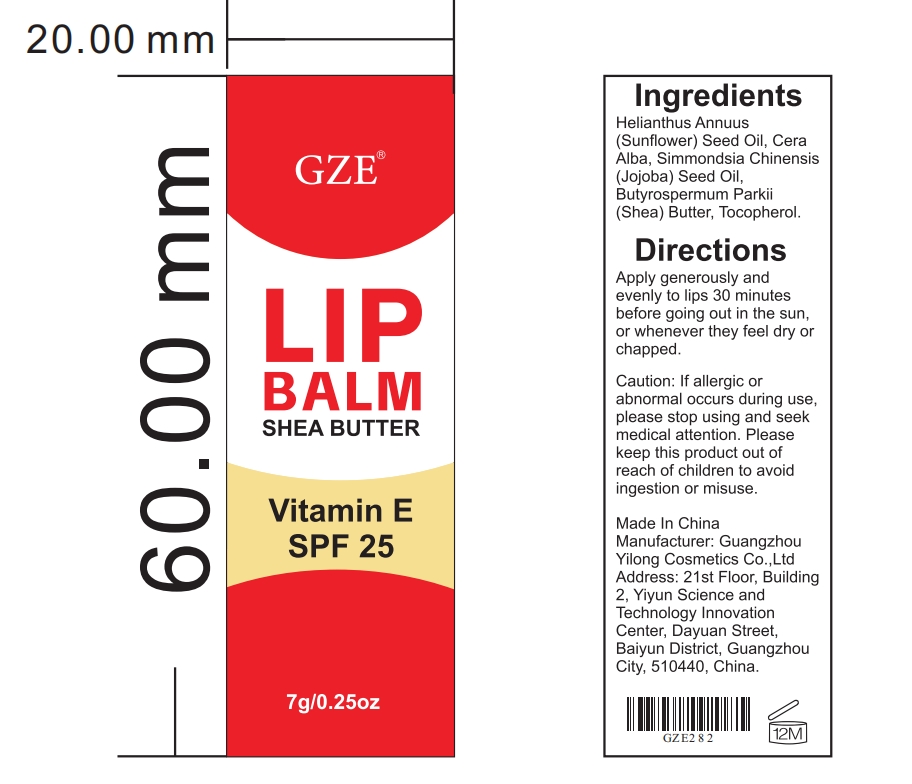
Label: GZE LIP BALM- sunflower seed oil,shea butter paste
- NDC Code(s): 83566-282-01
- Packager: Guangdong Aimu Biological Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated July 25, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- DO NOT USE
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GZE LIP BALM
sunflower seed oil,shea butter pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83566-282 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUNFLOWER SEED (UNII: R9N3379M4Z) (SUNFLOWER SEED - UNII:R9N3379M4Z) SUNFLOWER SEED 45 g in 100 g SHEA BUTTER (UNII: K49155WL9Y) (SHEA BUTTER - UNII:K49155WL9Y) SHEA BUTTER 15 g in 100 g Inactive Ingredients Ingredient Name Strength PEG-8 BEESWAX (UNII: 3C1QUF1TIR) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83566-282-01 7 g in 1 TUBE; Type 0: Not a Combination Product 07/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/19/2024 Labeler - Guangdong Aimu Biological Technology Co., Ltd (712647107) Establishment Name Address ID/FEI Business Operations Guangzhou Yilong Cosmetics Co., Ltd 712647107 manufacture(83566-282)