Label: TINNITUS RELINF PATCHES- camphor, borneol patch
- NDC Code(s): 84445-007-01
- Packager: Shenzhen Furuizhilian keji Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated July 17, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
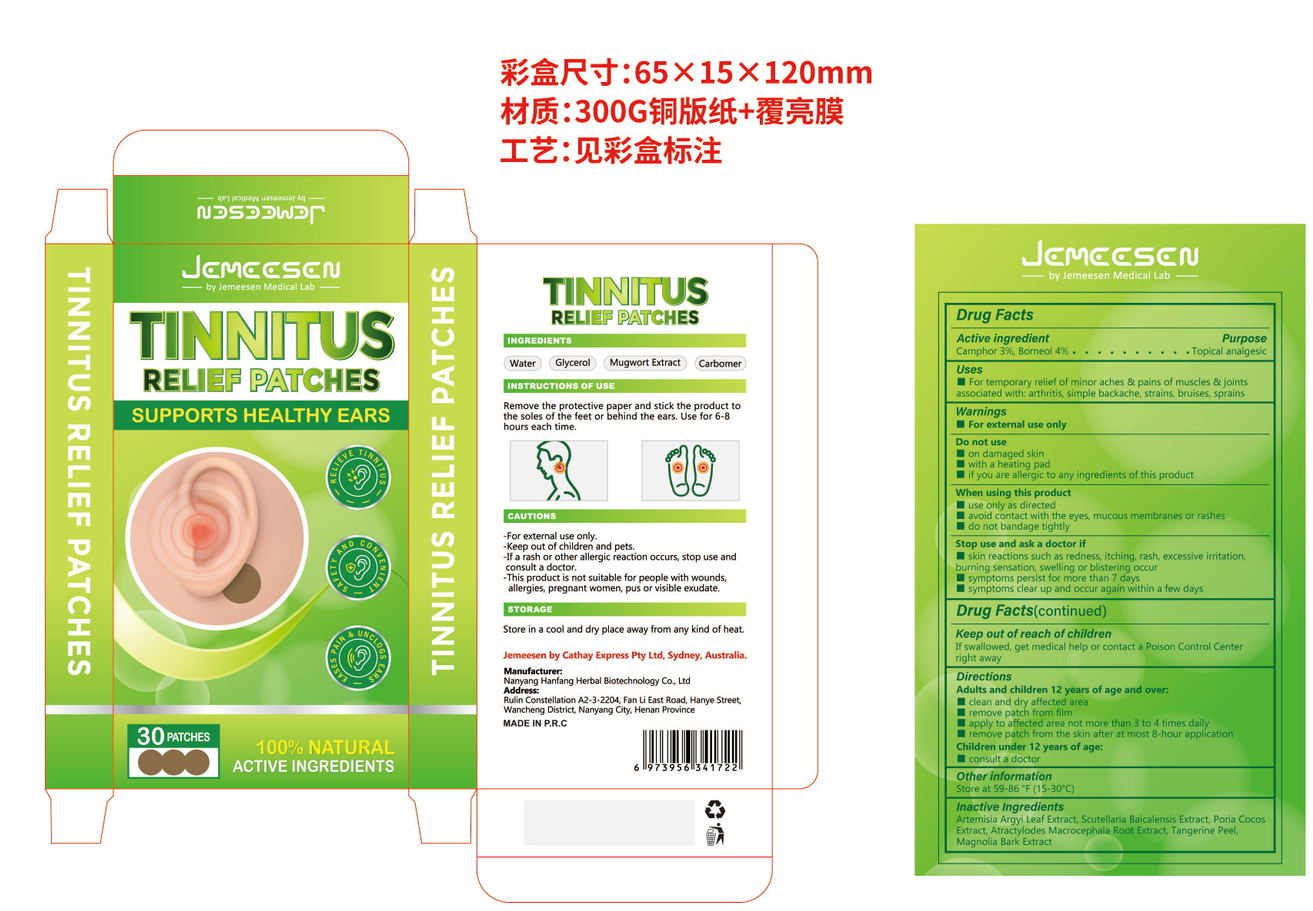
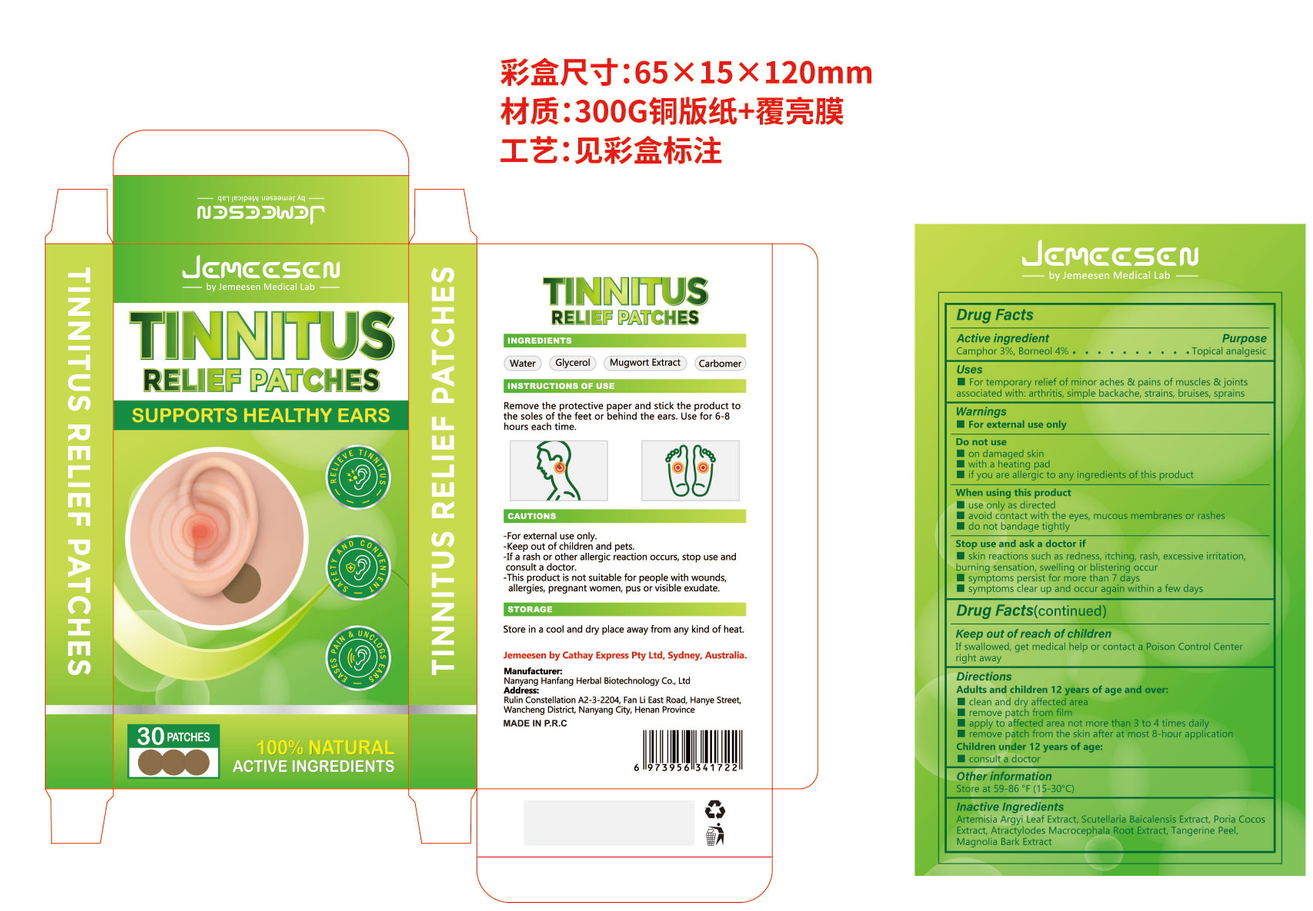
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TINNITUS RELINF PATCHES
camphor, borneol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84445-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BORNEOL (UNII: M89NIB437X) (BORNEOL - UNII:M89NIB437X) BORNEOL 2.4 g in 60 g CAMPHOR, (-)- (UNII: 213N3S8275) (CAMPHOR, (-)- - UNII:213N3S8275) CAMPHOR, (-)- 1.8 g in 60 g Inactive Ingredients Ingredient Name Strength FU LING (UNII: XH37TWY5O4) TANGERINE PEEL (UNII: JU3D414057) MAGNOLIA OBOVATA BARK (UNII: SM9Z2LD5TK) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) ATRACTYLODES MACROCEPHALA ROOT (UNII: 08T3N29QJB) ARTEMISIA ARGYI LEAF (UNII: 2JYC99Q0WZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84445-007-01 60 g in 1 BOX; Type 0: Not a Combination Product 07/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/17/2024 Labeler - Shenzhen Furuizhilian keji Co., Ltd. (418598613) Establishment Name Address ID/FEI Business Operations Shenzhen Furuizhilian keji Co., Ltd. 418598613 manufacture(84445-007)