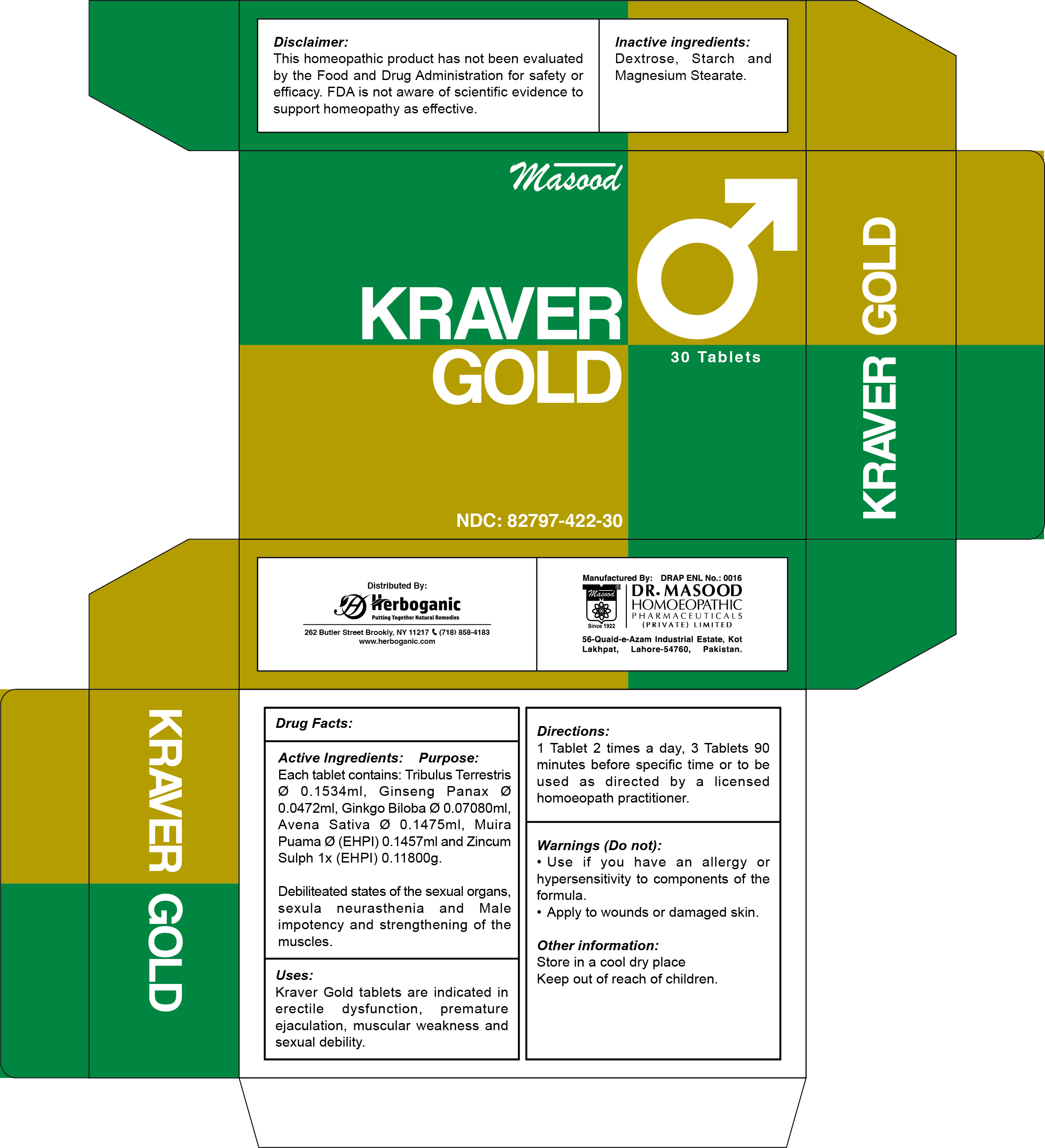
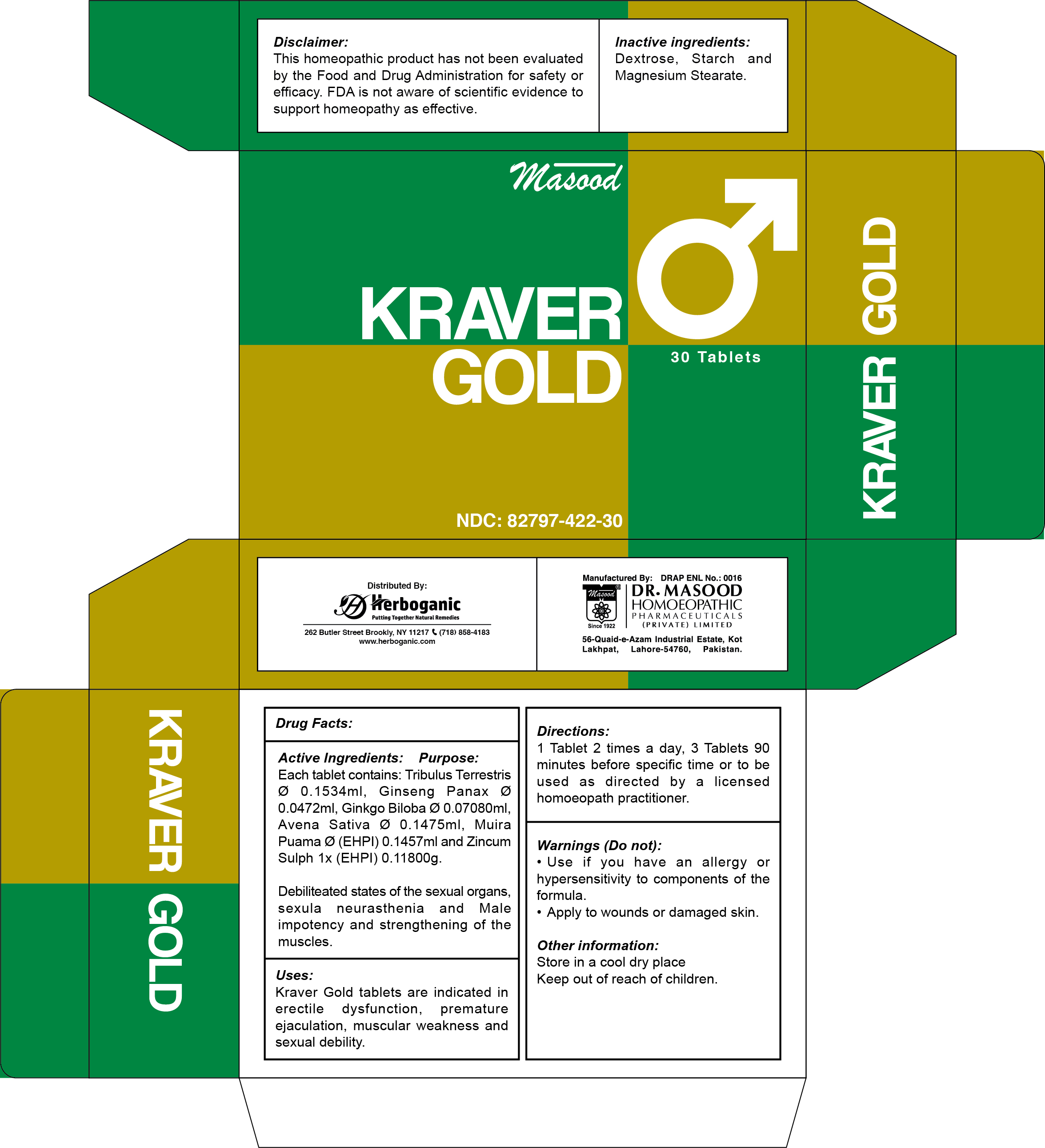
Label: KRAVER GOLD- tribulus terrestris, ginseng panax, ginkgo biloba, avena sativa, muira puama, zincum sulphate tablet, coated
- NDC Code(s): 82797-422-30
- Packager: Dr. Masood Homeopathic Pharmaceuticals Private Limited
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 9, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Dosage and Administration
- Indications and Usage
- Inactive Ingredients
- ACTIVE INGREDIENT
- Keep out of the reach of children
-
Purpose
Ingredient Purpose
TRIBULUS TERRESTRIS: Debilitated states of the sexual organs, sexual neurasthenia
GINSENG panax: Male impotency and strengthening of the muscles.
GINKGO BILOBA: Dilated arteries of male sexual organs by improving blood circulation to the body
AVENA SATIVA: Increases the male hormone (testosterone) level in the blood
MUIRA PUAMA: Considered a valuable remedy for impotence
ZINCUM SULPH: Muscles cramps. Hypochondriasis due to masturbation
- Warnings
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KRAVER GOLD
tribulus terrestris, ginseng panax, ginkgo biloba, avena sativa, muira puama, zincum sulphate tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82797-422 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC SULFATE (UNII: 89DS0H96TB) (ZINC CATION - UNII:13S1S8SF37) ZINC SULFATE 0.118 mg in 600 mg TRIBULUS TERRESTRIS WHOLE (UNII: 4X4HLN92OT) (TRIBULUS TERRESTRIS WHOLE - UNII:4X4HLN92OT) TRIBULUS TERRESTRIS WHOLE 0.1534 mg in 600 mg AVENA SATIVA WHOLE (UNII: 5P8D0Z74RG) (AVENA SATIVA WHOLE - UNII:5P8D0Z74RG) AVENA SATIVA WHOLE 0.1475 mg in 600 mg PTYCHOPETALUM OLACOIDES WHOLE (UNII: G582QI158H) (PTYCHOPETALUM OLACOIDES WHOLE - UNII:G582QI158H) PTYCHOPETALUM OLACOIDES WHOLE 0.1457 mg in 600 mg GINKGO BILOBA WHOLE (UNII: 660486U6OI) (GINKGO BILOBA WHOLE - UNII:660486U6OI) GINKGO BILOBA WHOLE 0.0708 mg in 600 mg PANAX GINSENG WHOLE (UNII: 9L5JEP7MES) (PANAX GINSENG WHOLE - UNII:9L5JEP7MES) PANAX GINSENG WHOLE 0.0472 mg in 600 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) 12 mg in 600 mg DEXTROSE (UNII: IY9XDZ35W2) 420 mg in 600 mg MAGNESIUM STEARATE (UNII: 70097M6I30) 6 mg in 600 mg Product Characteristics Color yellow (Golden) Score score with uneven pieces Shape ROUND Size 12mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82797-422-30 600 mg in 1 BLISTER PACK; Type 0: Not a Combination Product 07/09/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/09/2024 Labeler - Dr. Masood Homeopathic Pharmaceuticals Private Limited (645453119) Registrant - Dr. Masood Homeopathic Pharmaceuticals Private Limited (645453119) Establishment Name Address ID/FEI Business Operations Dr. Masood Homeopathic Pharmaceuticals Private Limited 645453119 manufacture(82797-422) , pack(82797-422) , label(82797-422)