Label: VITAMIN E VITALIZING SUNSCREEN cream
- NDC Code(s): 83872-270-01
- Packager: Shenzhen XiaoMai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated July 3, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
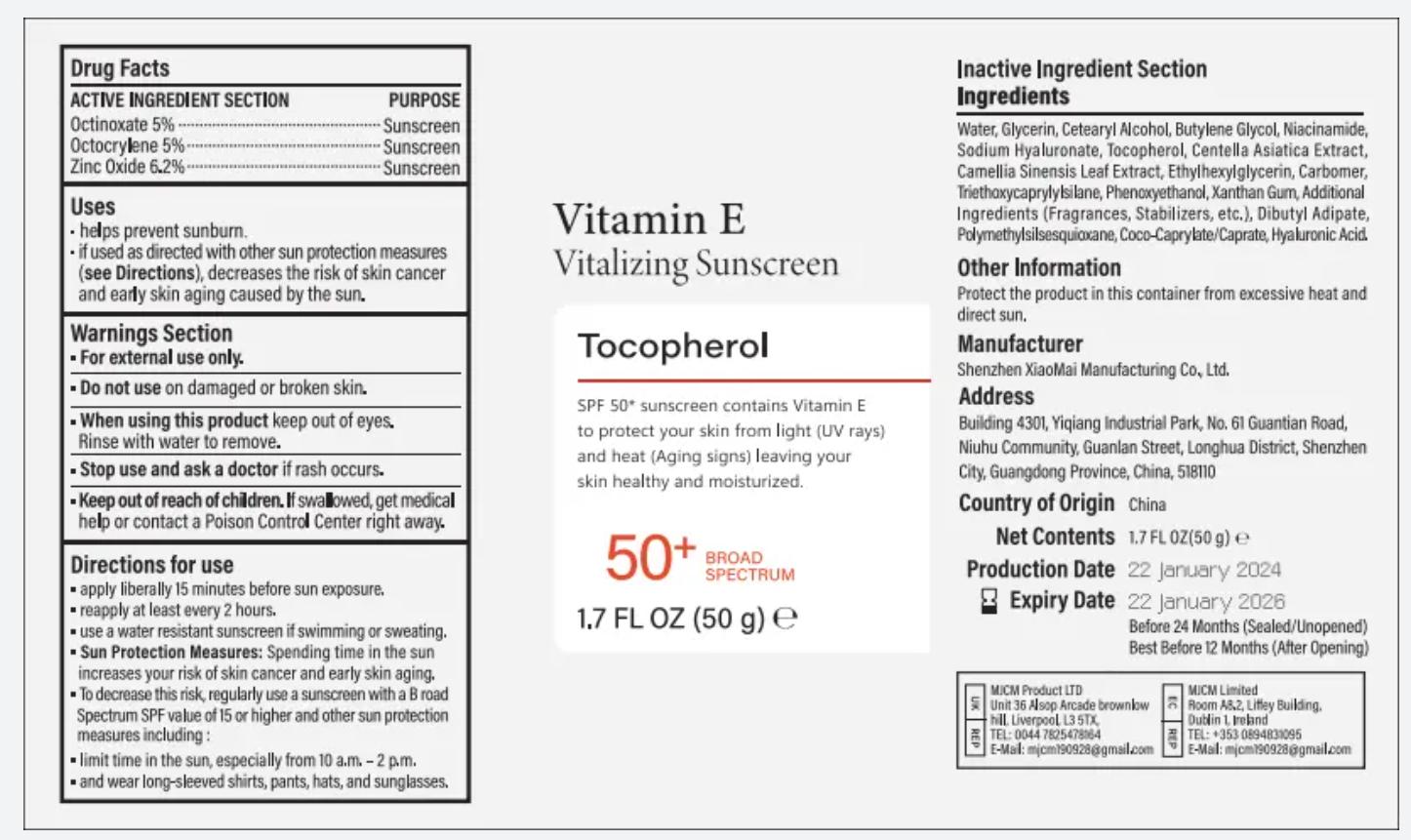
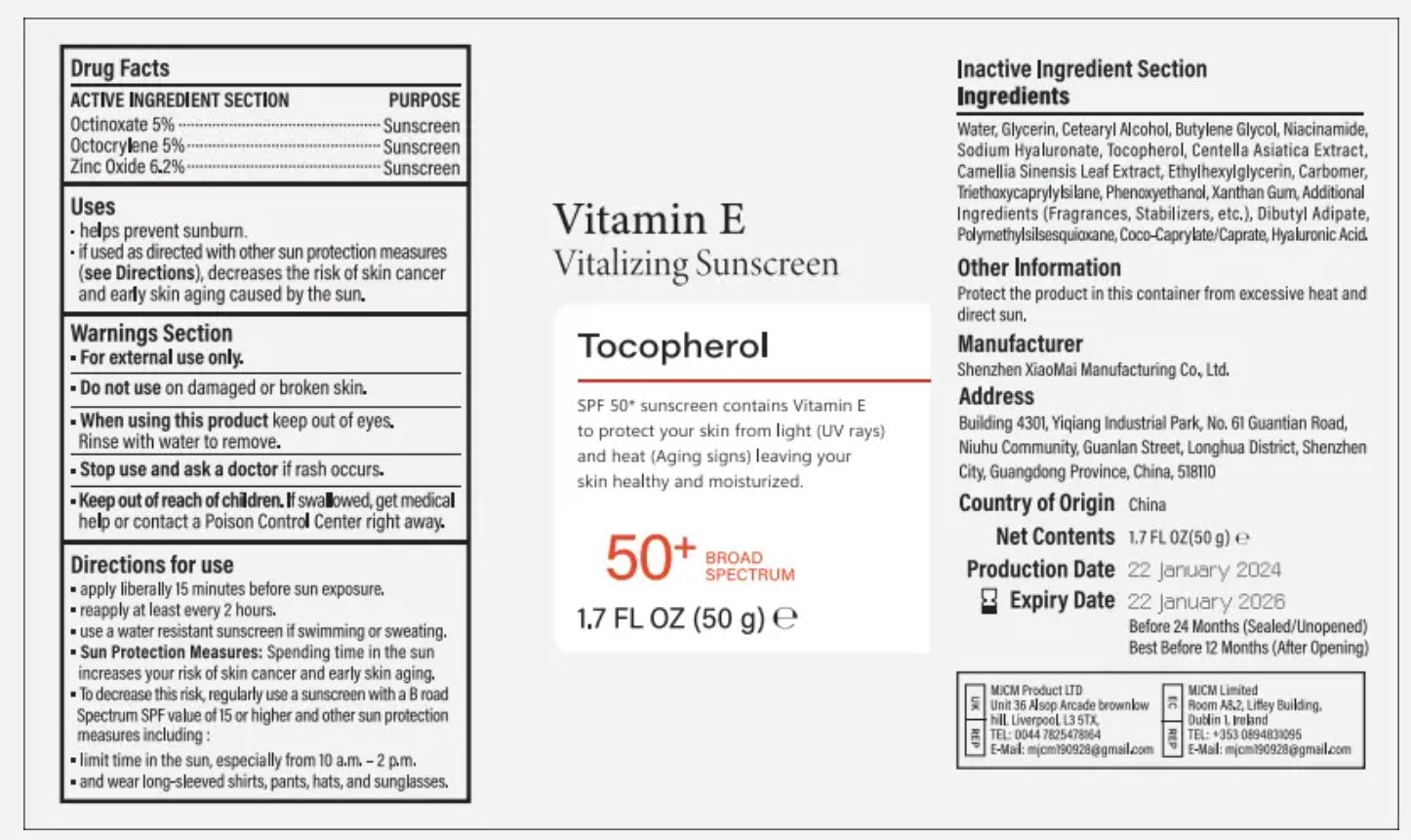
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children.
-
Directions for use
apply liberally 15 minutes before sun exposure,reapply at least every 2 hours.use a water resistant sunscreen if swimming or sweating.Sun Protection Measures: $pending time in the sunincreases your risk of skin cancer and early skin aging.To decrease this risk, reqularly use a sunscreen with a B roadSpectrum SPF value of 15 or higher and other sun protectionmeasures including :limit time in the sun, especially from 10 a.m. - 2 p.m.and wear longsleeved shirts, pants, hats, and sunglasses.
-
INACTIVE INGREDIENT
Water,
Glycerin,
Cetearyl Alcohol,
Butylene Glycol,
Niacinamide,
Sodium Hyaluronate,
Tocopherol,
Centella Asiatica Extract,
Camellia Sinensis Leaf Extract, Ethylhexylglycerin, CarbomerTriethaxycaprylylsilane, Phenoxyethanol,
Xanthan Gum, AdditionalIngredients (Fragrances, Stabilizers, etc,), Dibutyl Adipate,
Polymethylsilsesquioxane,
Coco-Caprylate'Caprate, Hyaluronic Acid. - Other Information
- label
-
INGREDIENTS AND APPEARANCE
VITAMIN E VITALIZING SUNSCREEN
vitamin e vitalizing sunscreen creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-270 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 100 g in 1 mg Inactive Ingredients Ingredient Name Strength AVOCADO FRUIT WATER (UNII: QY52B5ZP10) 100 g in 1 mg OCTINOXATE (UNII: 4Y5P7MUD51) 100 g in 1 mg .ALPHA.-TOCOPHEROL CALCIUM SUCCINATE, D- (UNII: BVK87L5TNB) 100 g in 1 mg PEG-9 DIGLYCIDYL ETHER/SODIUM HYALURONATE CROSSPOLYMER (UNII: 788QAG3W8A) 100 g in 1 mg OCTOCRYLENE (UNII: 5A68WGF6WM) 100 g in 1 mg CETEARYL BEHENATE (UNII: 7ARI9LTH0U) 100 g in 1 mg ISONIACINAMIDE (UNII: 4H3BH6YX9Q) 100 g in 1 mg 1-BUTYLGLYCERIN (UNII: 8834DFG5HH) 100 g in 1 mg 1,3-BUTYLENE GLYCOL 1-PROPIONATE (UNII: 17U77WTV66) 100 g in 1 mg Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-270-01 50 mg in 1 BOTTLE; Type 0: Not a Combination Product 07/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/03/2024 Labeler - Shenzhen XiaoMai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen XiaoMai Manufacturing Co., Ltd. 712999147 manufacture(83872-270)