Label: NALOXONE HYDROCHLORIDE spray
- NDC Code(s): 80425-0409-1
- Packager: Advanced Rx of Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 45802-578
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 27, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each spray)
- Purpose
- Uses
- KEEP OUT OF REACH OF CHILDREN
-
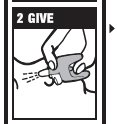
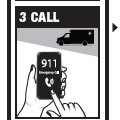
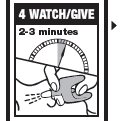
Directions
- Warning
- Other information
- Inactive Ingredients
- Questions?
-
Package/Label Principal Display Panel
NDC: 80425-0409-01
Naloxone HCl Nasal Spray 4 mg
Emergency Treatment of Opioid Overdose
Original Prescription Strength
Easy to Use
Can Save a Life
Designed to Rapidly Reverse the Effects of a Life-Threatening Opioid Emergency
For use in nose only
2 Single-Dose Nasal Spray Devices
0.003 fl oz (0.1mL) each

-
INGREDIENTS AND APPEARANCE
NALOXONE HYDROCHLORIDE
naloxone hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80425-0409(NDC:45802-578) Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80425-0409-1 2 in 1 CARTON 06/27/2024 1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211951 06/27/2024 Labeler - Advanced Rx of Tennessee, LLC (117023142) Establishment Name Address ID/FEI Business Operations Advanced Rx of Tennessee, LLC 117023142 repack(80425-0409)