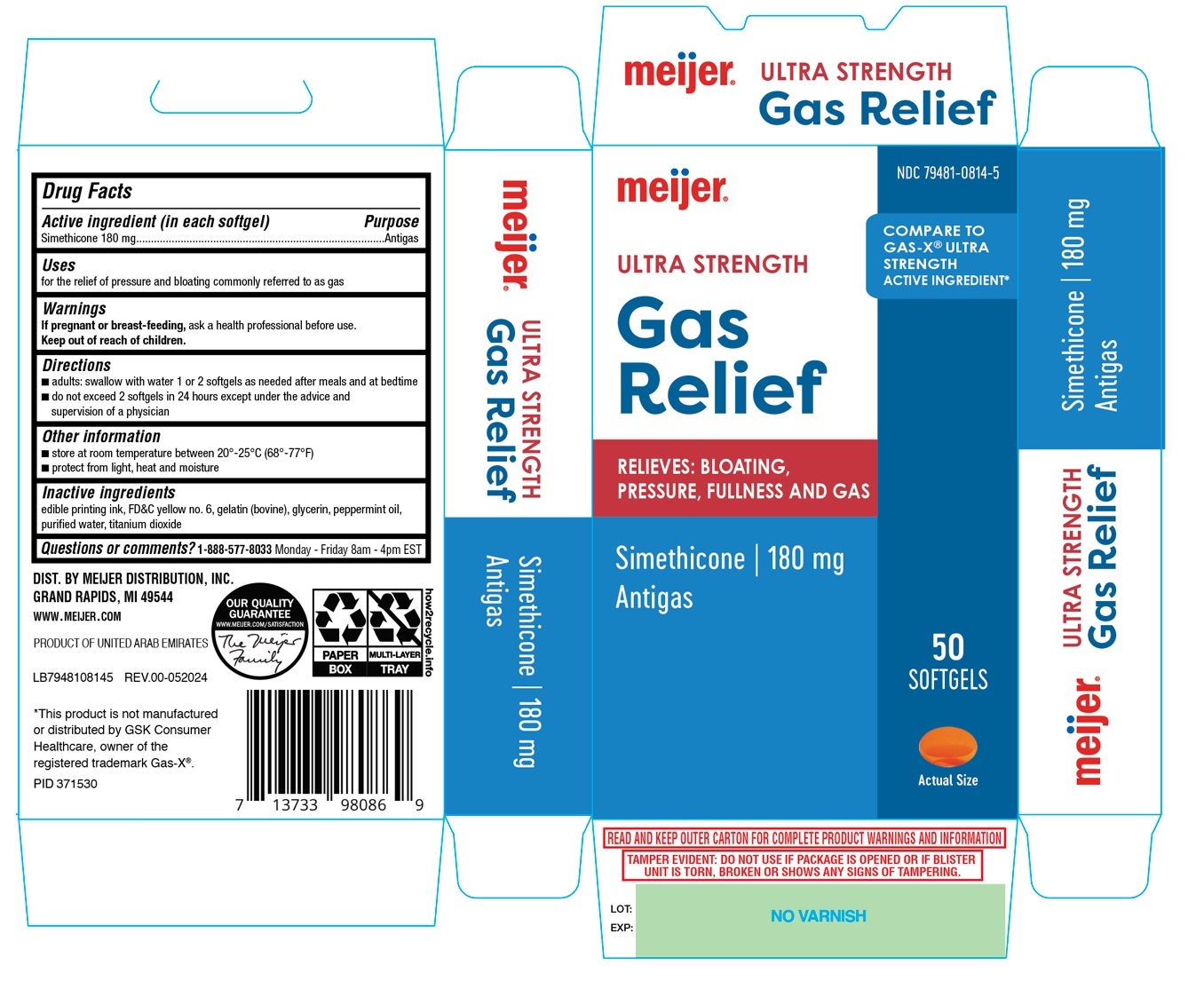
Label: MEIJER ULTRA STRENGTH GAS RELIEF SOFTGEL- simethicone capsule, liquid filled
- NDC Code(s): 79481-0814-5
- Packager: Meijer, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 6, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each softgel)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
COMPARE TO GAS-X® ULTRA STRENGHT ACTIVE INGREDIENT*
RELIEVES: BLOATING,
PRESSURE, FULLNESS AND GAS
DIST. BY MEIJER DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544WWW.MEIJER.COM
PRODUCT OF UNITED ARAB EMIRATES
*This product is not manufactured or distributed by GSK consumer Healthcare, owner of the registered trademark GAS-X®
READ AND KEEP OUTER CARTON FOR COMPLETE PRODUCT WARNINGS AND INFORMATION TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING. - Packaging
-
INGREDIENTS AND APPEARANCE
MEIJER ULTRA STRENGTH GAS RELIEF SOFTGEL
simethicone capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-0814 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE, UNSPECIFIED 180 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN TYPE B BOVINE (230 BLOOM) (UNII: WIL1404U79) GLYCERIN (UNII: PDC6A3C0OX) PEPPERMINT OIL (UNII: AV092KU4JH) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape OVAL Size 12mm Flavor Imprint Code 814 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-0814-5 2 in 1 CARTON 06/27/2024 1 25 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 06/27/2024 Labeler - Meijer, Inc. (006959555)