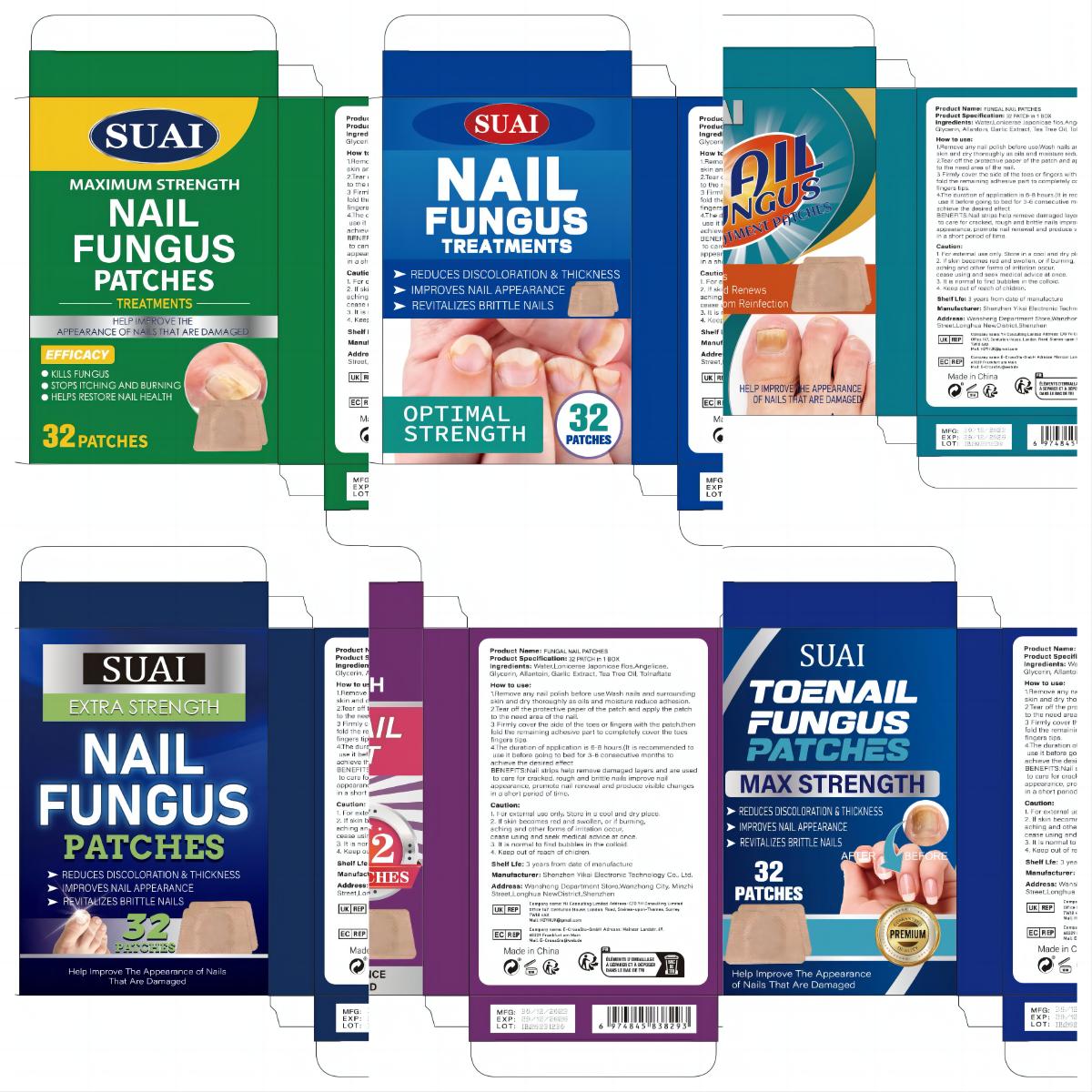
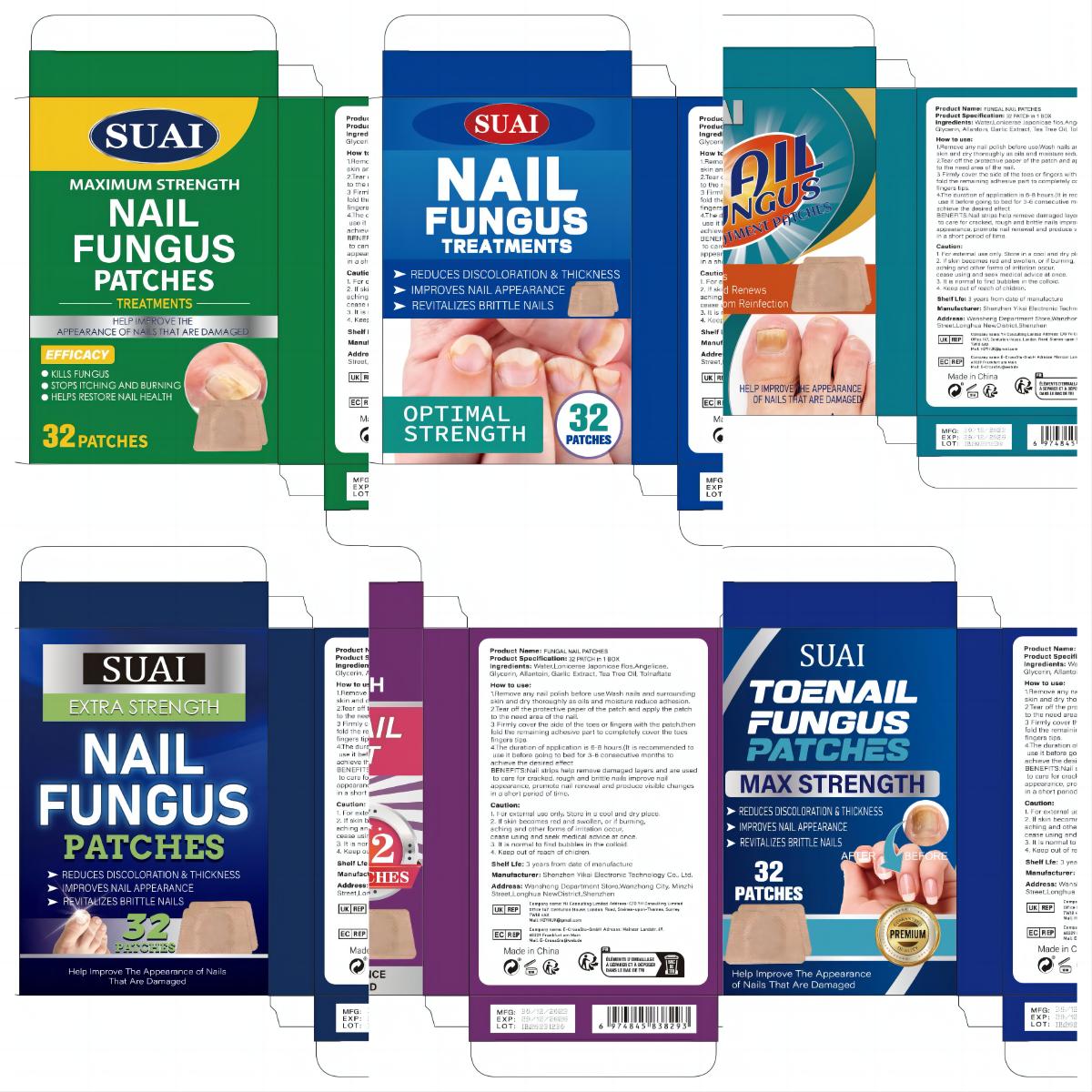
Label: FUNGAL NAIL PATCHES patch
- NDC Code(s): 83809-021-01
- Packager: Shenzhen Yikai Electronic Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 13, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
Use
1.Remove any nail polish before use.Wash nails and surrounding skin and dry thoroughly as oils and moisture reduce adhesion.
2.Tear off the protective paper of the patch and apply the patch to the need area of the nail.
3 Firmly cover the side of the toes or fingers with the patch.then fold the remaining adhesive part to completely cover the toes fingers tips.
4.The duration of application is 6-8 hours.(lt is recommended to use it before going to bed for 3-6 consecutive months to achieve the desired effect. - Warnings
- Stop Use
- Keep Oot Of Reach Of Children
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FUNGAL NAIL PATCHES
fungal nail patches patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83809-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 Inactive Ingredients Ingredient Name Strength TEA TREE OIL (UNII: VIF565UC2G) WATER (UNII: 059QF0KO0R) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) GARLIC (UNII: V1V998DC17) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83809-021-01 32 in 1 BOX; Type 0: Not a Combination Product 01/08/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 01/08/2024 Labeler - Shenzhen Yikai Electronic Technology Co., Ltd. (700426808) Establishment Name Address ID/FEI Business Operations Shenzhen Yikai Electronic Technology Co., Ltd. 700426808 label(83809-021) , manufacture(83809-021)