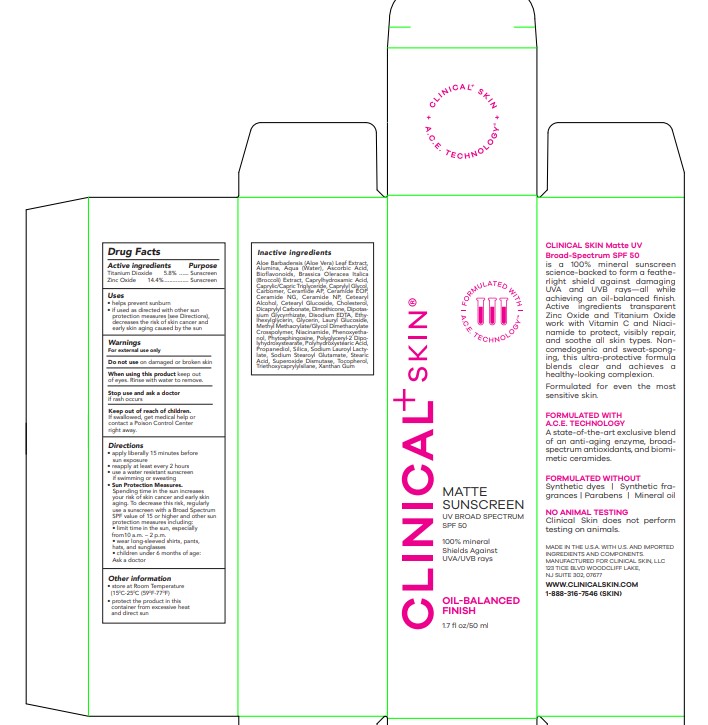
Label: MATTE SUNSCREEN SPF 50- titanium dioxide, zinc oxide lotion
- NDC Code(s): 84248-025-01
- Packager: CLINICAL SKIN LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Use
- Warnings
-
Directions
• apply liberally 15 minutes before sun exposure
• reapply at least every 2 hours • use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
• children under 6 months of age: Ask a doctor
- Other Information
-
Inactive ingredients
Aloe Barbadensis (Aloe Vera) Leaf Extract, Alumina, Aqua (Water), Ascorbic Acid, Bioflavonoids, Brassica Oleracea Italica (Broccoli) Extract, Caprylhydroxamic Acid, Caprylic/Capric Triglyceride, Caprylyl Glycol, Carbomer, Ceramide AP, Ceramide EOP, Ceramide NG, Ceramide NP, Cetearyl Alcohol, Cetearyl Glucoside, Cholesterol, Dicaprylyl Carbonate, Dimethicone, Dipotassium Glycyrrhizate, Disodium EDTA, Ethylhexylglycerin, Glycerin, Lauryl Glucoside, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Niacinamide, Phenoxyethanol, Phytosphingosine, Polyglyceryl-2 Dipolyhydroxystearate, Polyhydroxystearic Acid, Propanediol, Silica, Sodium Lauroyl Lactylate, Sodium Stearoyl Glutamate, Stearic Acid, Superoxide Dismutase, Tocopherol, Triethoxycaprylylsilane, Xanthan Gum
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MATTE SUNSCREEN SPF 50
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84248-025 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14.4 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 5.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength BROCCOLI SEED OIL (UNII: SY01LVD4G4) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PHENOXYETHANOL (UNII: HIE492ZZ3T) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) GLYCERIN (UNII: PDC6A3C0OX) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CERAMIDE NG (UNII: C04977SRJ5) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) CITRUS BIOFLAVONOIDS (UNII: BD70459I50) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) EDETATE DISODIUM (UNII: 7FLD91C86K) CHOLESTEROL (UNII: 97C5T2UQ7J) ASCORBIC ACID (UNII: PQ6CK8PD0R) ALUMINUM OXIDE (UNII: LMI26O6933) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MUSKMELON (UNII: ZV095H5633) NIACINAMIDE (UNII: 25X51I8RD4) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PROPANEDIOL (UNII: 5965N8W85T) STEARIC ACID (UNII: 4ELV7Z65AP) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) XANTHAN GUM (UNII: TTV12P4NEE) CERAMIDE AP (UNII: F1X8L2B00J) CERAMIDE NP (UNII: 4370DF050B) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84248-025-01 1 in 1 CARTON 06/17/2024 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/17/2024 Labeler - CLINICAL SKIN LLC (106954468) Registrant - CLINICAL SKIN LLC (106954468)