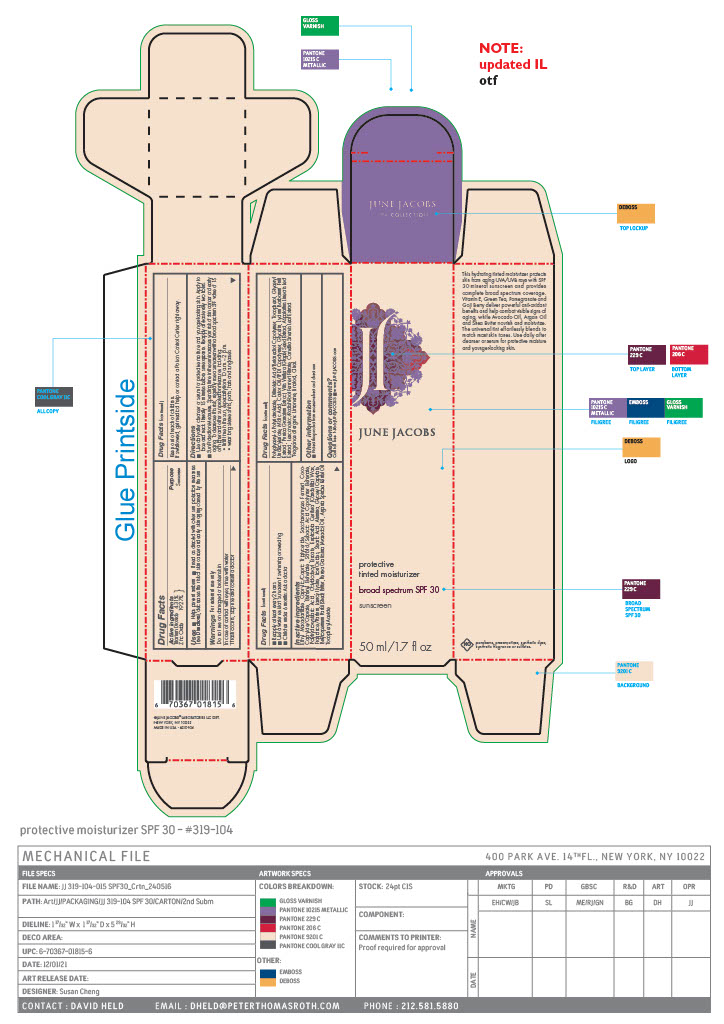
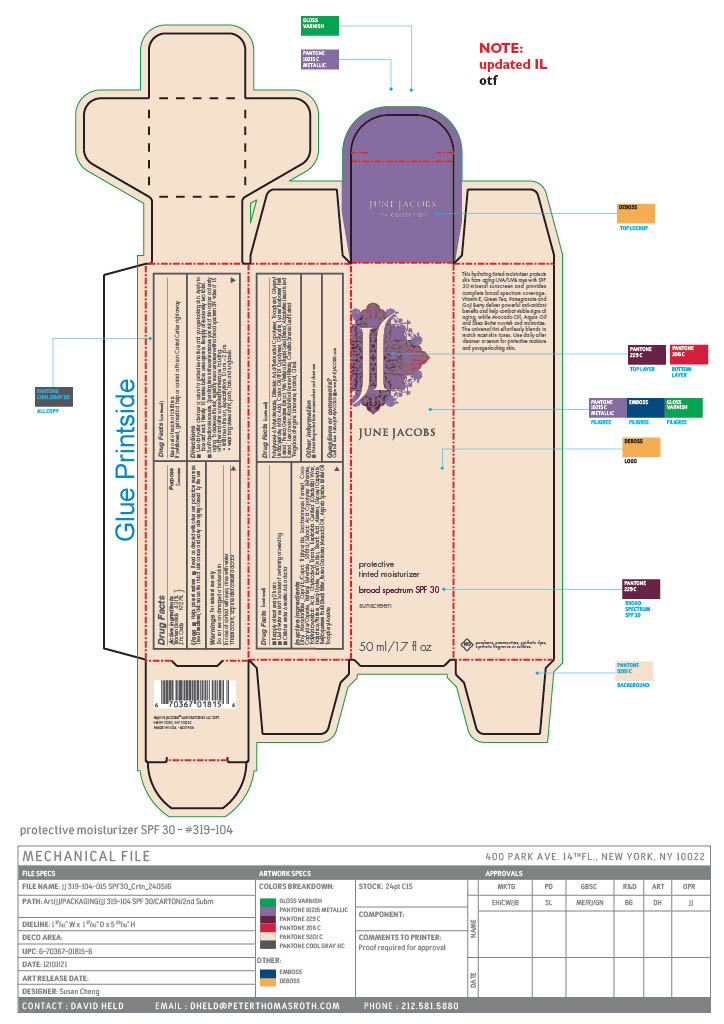
Label: PROTECTIVE TINTED MOISTURIZER BROAD SPECTRUM SUNSCREEN SPF 30- titanium dioxide, zinc oxide paste
- NDC Code(s): 65278-104-01, 65278-104-02
- Packager: June Jacobs Labs LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 20, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- Use daily after cleanser or serum for protective moisture and younger-looking skin. Apply to face and neck liberally 15 minutes before sun exposure. Reapply at least every two hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. – 2 p.m. • wear long sleeve shirts, pants, hats and sunglasses
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months: Ask a doctor
-
INACTIVE INGREDIENT
Inactive Ingredients
Ethyl Macadamiate, Caprylic/Capric Triglyceride, Saccharomyces Ferment, Coco Caprylate/Caprate, Behenyl Behenate, Sorbitol/Sebacic Acid Copolymer Behenate, Polyhydroxystearic Acid, Octyldodecyl Erucate, Euphorbia Cerifera (Candelilla) Wax, Fragrance/Parfum, Lauroyl Lysine, Iron Oxides, Stearic Acid, Alumina, Glyceryl Caprylate, Butyrospermum Parkii (Shea) Butter, Persea Gratissima (Avocado) Oil , Argania Spinosa Kernel Oil, Tocopheryl Acetate, Polyglyceryl-6 Polyricinoleate, Dilinoleic Acid/Butanediol Copolymer, Tocopherol, Glyceryl Undecylenate, Malic Acid, Castor Oil/IPDI Copolymer, Glycerin, Lycium Barbarum Fruit Extract , Punica Granatum Extract, Vitis Vinifera (Grape) Seed Extract, Aspalathus Linearis Leaf Extract , Leuconostoc/Radish Root Ferment Filtrate, Camellia Sinensis Leaf Extract, Limonene, Linalool, Citral.
- OTHER SAFETY INFORMATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROTECTIVE TINTED MOISTURIZER BROAD SPECTRUM SUNSCREEN SPF 30
titanium dioxide, zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65278-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 9.693 g in 50.28 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.169 g in 50.28 g Inactive Ingredients Ingredient Name Strength GREEN TEA LEAF (UNII: W2ZU1RY8B0) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) SUNFLOWER OIL (UNII: 3W1JG795YI) ARGAN OIL (UNII: 4V59G5UW9X) MALIC ACID (UNII: 817L1N4CKP) GLYCERIN (UNII: PDC6A3C0OX) PUNICA GRANATUM ROOT BARK (UNII: CLV24I3T1D) LAUROYL LYSINE (UNII: 113171Q70B) JUNIPER BERRY OIL (UNII: SZH16H44UY) ASPALATHUS LINEARIS LEAF (UNII: H7UGK1GJCU) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) SHEA BUTTER (UNII: K49155WL9Y) ETHYL MACADAMIATE (UNII: ANA2NCS6V1) LITSEA OIL (UNII: 2XIW34BN6O) GINGER OIL (UNII: SAS9Z1SVUK) TOCOPHEROL (UNII: R0ZB2556P8) GLYCERYL 1-UNDECYLENATE (UNII: B68LJT9544) CASTOR OIL (UNII: D5340Y2I9G) LYCIUM BARBARUM FRUIT (UNII: 930626MWDL) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALUMINUM OXIDE (UNII: LMI26O6933) AVOCADO OIL (UNII: 6VNO72PFC1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) DILINOLEIC ACID/BUTANEDIOL COPOLYMER (UNII: 1F2S8T535O) LAVANDIN OIL (UNII: 9RES347CKG) VETIVER OIL (UNII: 9M9P32M01L) FERRIC OXIDE RED (UNII: 1K09F3G675) STEARIC ACID (UNII: 4ELV7Z65AP) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) OCTYLDODECYL ERUCATE (UNII: D4N66T98C2) AMYRIS BALSAMIFERA OIL (UNII: I1BJ961J2E) GERANIUM OIL, ALGERIAN TYPE (UNII: 5Q1I94P4WG) POGOSTEMON CABLIN LEAF OIL (UNII: F3IN55X5PO) GALBANUM (UNII: C690YC9N2Z) CITRAL (UNII: T7EU0O9VPP) BEHENYL BEHENATE (UNII: K8NU647RJ0) CANDELILLA WAX (UNII: WL0328HX19) ORANGE OIL (UNII: AKN3KSD11B) VITIS VINIFERA SEED (UNII: C34U15ICXA) Product Characteristics Color brown (Nude/Beige) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65278-104-01 50.28 g in 1 TUBE; Type 0: Not a Combination Product 09/08/2021 2 NDC:65278-104-02 50.28 g in 1 CARTON; Type 0: Not a Combination Product 09/08/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/08/2021 Labeler - June Jacobs Labs LLC (082439410) Establishment Name Address ID/FEI Business Operations June Jacobs Labs, LLC 122610681 manufacture(65278-104)