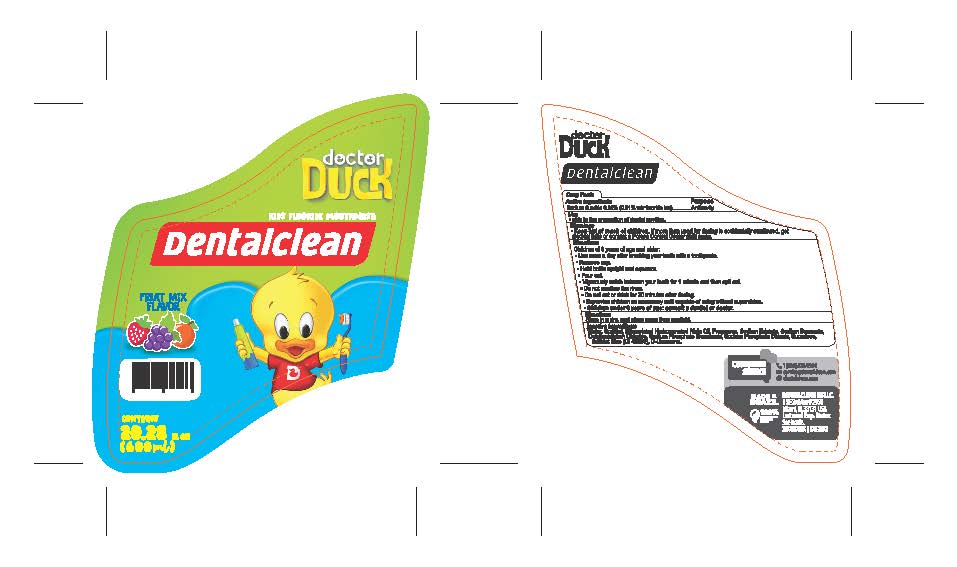
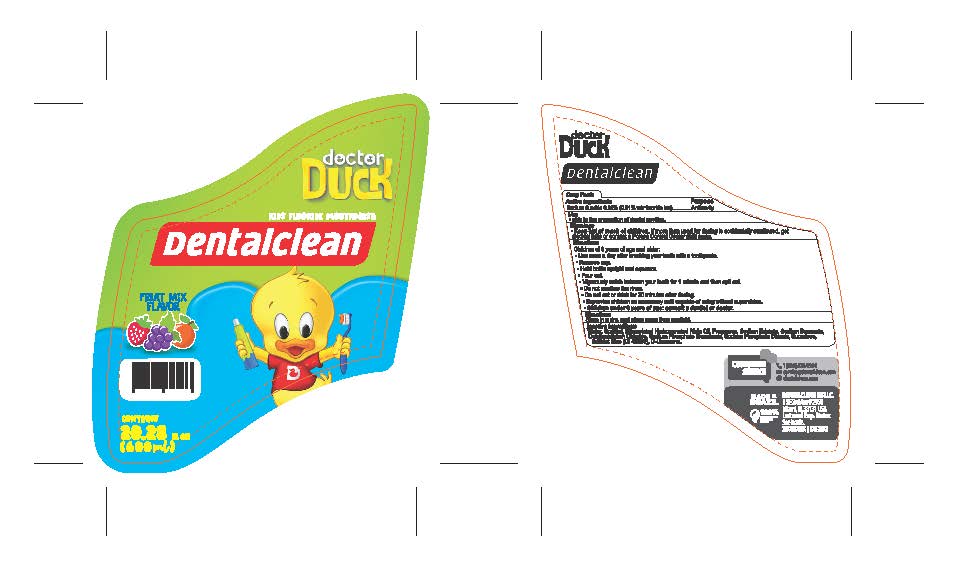
Label: DENTALCLEAN DOCTOR DUCK KIDS FLOURIDE MOUTHWASH- sodium fluoride 0.02% rinse
- NDC Code(s): 84035-010-20
- Packager: Rabbit Indústria e Comércio de Produtos de Higiene Pessoal Ltda
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 15, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
Directions
Children of 6 years of age and older:
• Use once a day after brushing your teeth with a toothpaste.
•Remove cap
• Hold bottle upright and squeeze
•Pour out
•Vigorously swish between your teeth for 1 minute and then spit out
• Do not swallow the rinse.
• Do not eat or drink for 30 minutes after rinsing.
• Supervise children as necessary until capable of using without supervision.
• Children under 6 years of age: consult a dentist or doctor. - OTHER SAFETY INFORMATION
- Inactive Ingredients
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DENTALCLEAN DOCTOR DUCK KIDS FLOURIDE MOUTHWASH
sodium fluoride 0.02% rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84035-010 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SUCRALOSE (UNII: 96K6UQ3ZD4) CETYLPYRIDINIUM CHLORIDE ANHYDROUS (UNII: 6BR7T22E2S) TETRASODIUM EDETATE DIHYDRATE (UNII: 3JGX4KKZ4A) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SORBITOL (UNII: 506T60A25R) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM PHOSPHATE DIBASIC DIHYDRATE (UNII: 94255I6E2T) LIMONENE, (+/-)- (UNII: 9MC3I34447) POLYOXYL 60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) Product Characteristics Color Score Shape Size Flavor TUTTI FRUTTI Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84035-010-20 600 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 06/01/2024 Labeler - Rabbit Indústria e Comércio de Produtos de Higiene Pessoal Ltda (901719567) Establishment Name Address ID/FEI Business Operations Rabbit Indústria e Comércio de Produtos de Higiene Pessoal Ltda 901719567 manufacture(84035-010)