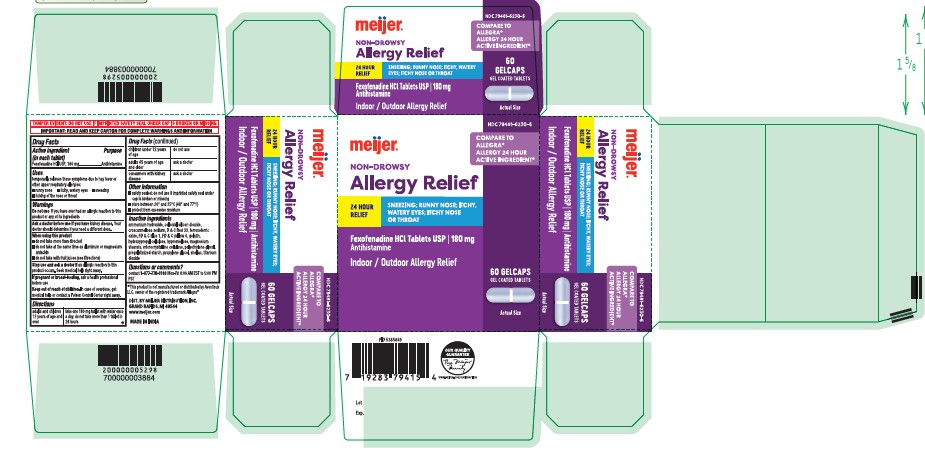
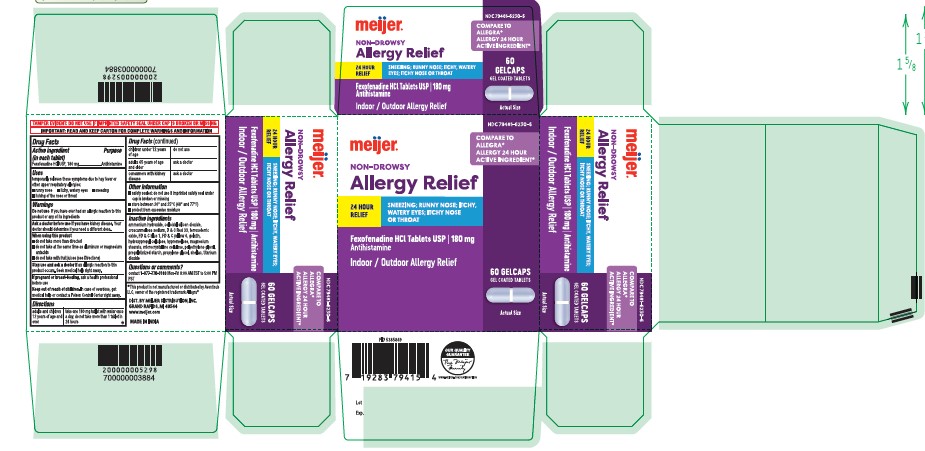
Label: FEXOFENADINE HCL tablet
- NDC Code(s): 79481-6230-6
- Packager: Meijer, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 7, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each tablet)
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HCL
fexofenadine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-6230 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 28 (UNII: 767IP0Y5NH) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) GELATIN (UNII: 2G86QN327L) HYPROMELLOSES (UNII: 3NXW29V3WO) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 33 (UNII: 9DBA0SBB0L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) Product Characteristics Color gray (White band in the middle) Score no score Shape OVAL (capsule shaped tablet) Size 20mm Flavor Imprint Code G18 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-6230-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211075 07/01/2024 Labeler - Meijer, Inc. (006959555)