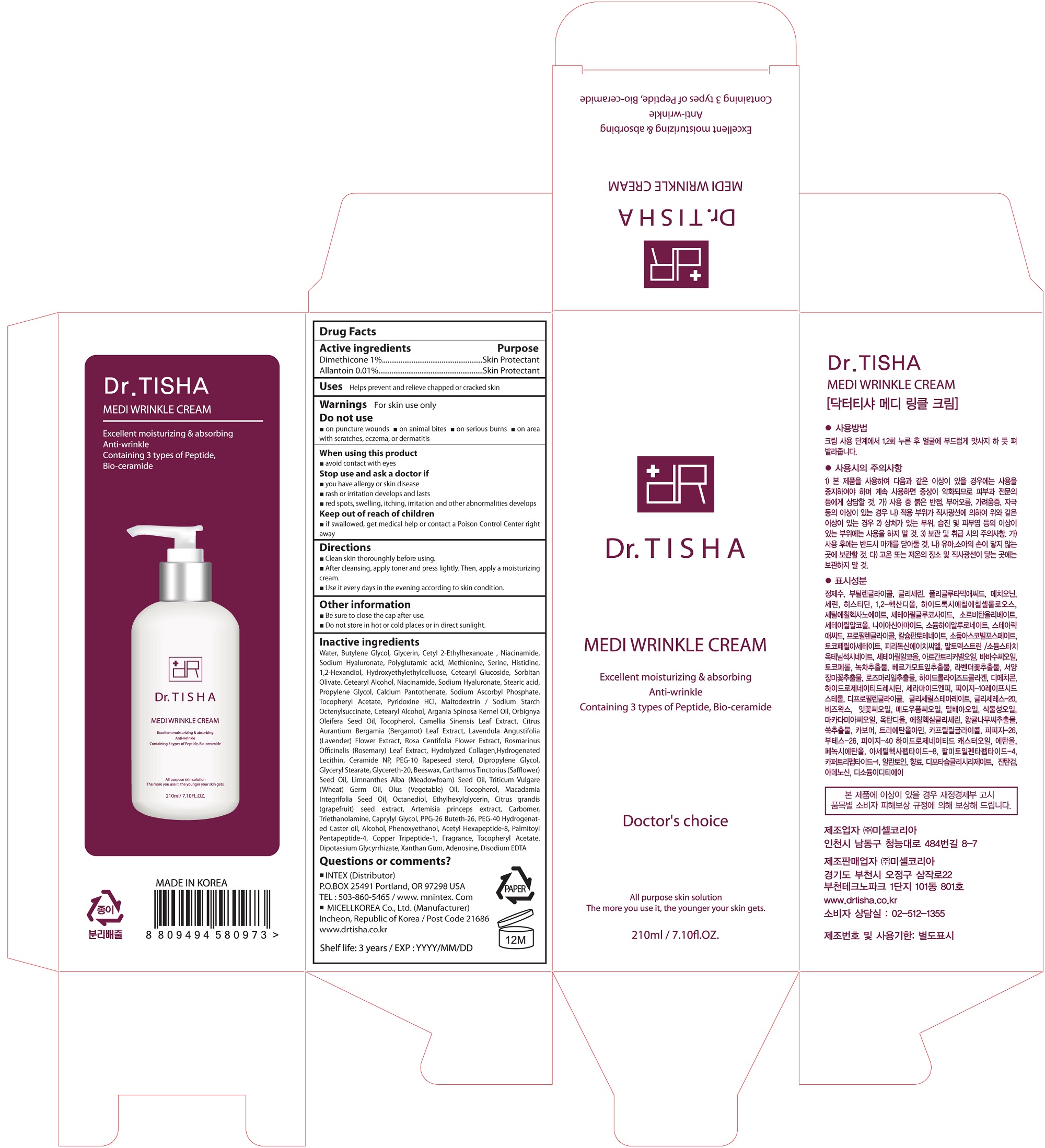
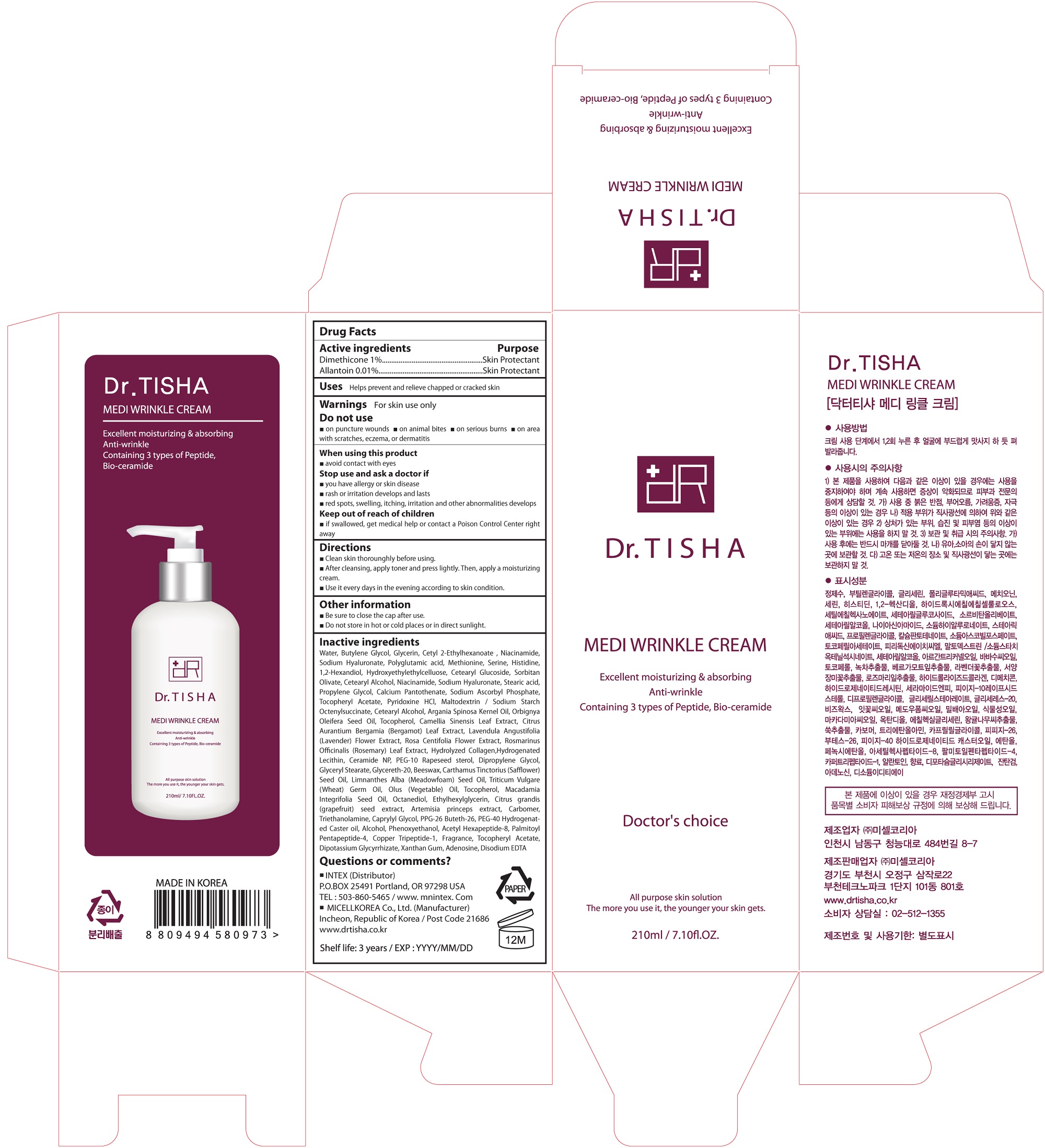
Label: MEDI WRINKLE CREAM- dimethicone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 71327-004-01 - Packager: MICELL KOREA CO.,LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 23, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Water, Butylene Glycol, Glycerin, Cetyl 2-Ethylhexanoate , Niacinamide, Sodium Hyaluronate, Polyglutamic acid, Methionine, Serine, Histidine, 1,2-Hexandiol, Hydroxyethylethylcelluose, Cetearyl Glucoside, Sorbitan Olivate, Cetearyl Alcohol, Niacinamide, Sodium Hyaluronate, Stearic acid, Propylene Glycol, Calcium Pantothenate, Sodium Ascorbyl Phosphate, Tocopheryl Acetate, Pyridoxine HCl, Maltodextrin / Sodium Starch Octenylsuccinate, Cetearyl Alcohol, Argania Spinosa Kernel Oil, Orbignya Oleifera Seed Oil, Tocopherol, Camellia Sinensis Leaf Extract, Citrus Aurantium Bergamia (Bergamot) Leaf Extract, Lavendula Angustifolia (Lavender) Flower Extract, Rosa Centifolia Flower Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Hydrolyzed Collagen,Hydrogenated Lecithin, Ceramide NP, PEG-10 Rapeseed sterol, Dipropylene Glycol, Glyceryl Stearate, Glycereth-20, Beeswax, Carthamus Tinctorius (Safflower) Seed Oil, Limnanthes Alba (Meadowfoam) Seed Oil, Triticum Vulgare (Wheat) Germ Oil, Olus (Vegetable) Oil, Tocopherol, Macadamia Integrifolia Seed Oil, Octanediol, Ethylhexylglycerin, Citrus grandis (grapefruit) seed extract, Artemisia princeps extract, Carbomer, Triethanolamine, Caprylyl Glycol, PPG-26 Buteth-26, PEG-40 Hydrogenated Caster oil, Alcohol, Phenoxyethanol, Acetyl Hexapeptide-8, Palmitoyl Pentapeptide-4, Copper Tripeptide-1, Fragrance, Tocopheryl Acetate, Dipotassium Glycyrrhizate, Xanthan Gum, Adenosine, Disodium EDTA
- PURPOSE
-
WARNINGS
For skin use only.
Do not use
on puncture wounds on animal bites on serious burns on area with scratches, eczema, or dermatitis
When using this product
avoid contact with eyes
Stop use and ask a doctor if
you have allergy or skin disease
rash or irritation develops and lasts
red spots, swelling, itching, irritation and other abnormalities develops - KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEDI WRINKLE CREAM
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71327-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dimethicone (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) Dimethicone 0.0021 g in 210 mL Allantoin (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) Allantoin 0.000021 g in 210 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Methionine (UNII: AE28F7PNPL) Serine (UNII: 452VLY9402) Histidine (UNII: 4QD397987E) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Maltodextrin (UNII: 7CVR7L4A2D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71327-004-01 210 mL in 1 PACKAGE; Type 0: Not a Combination Product 03/20/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/20/2017 Labeler - MICELL KOREA CO.,LTD (689851542) Registrant - MICELL KOREA CO.,LTD (689851542) Establishment Name Address ID/FEI Business Operations MICELL KOREA CO.,LTD 689851542 manufacture(71327-004)