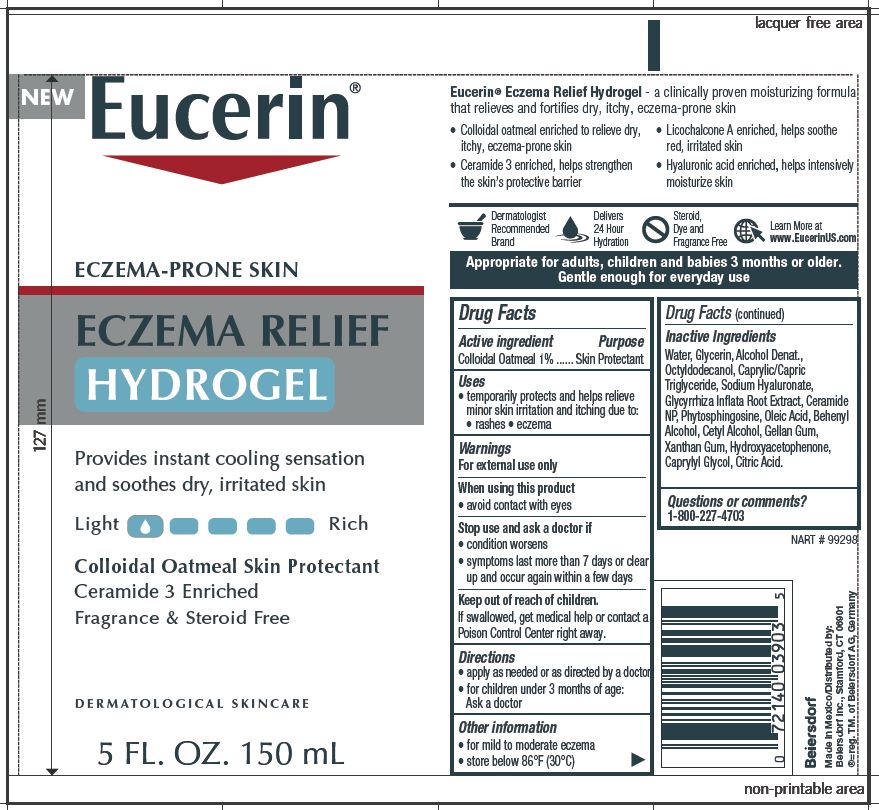
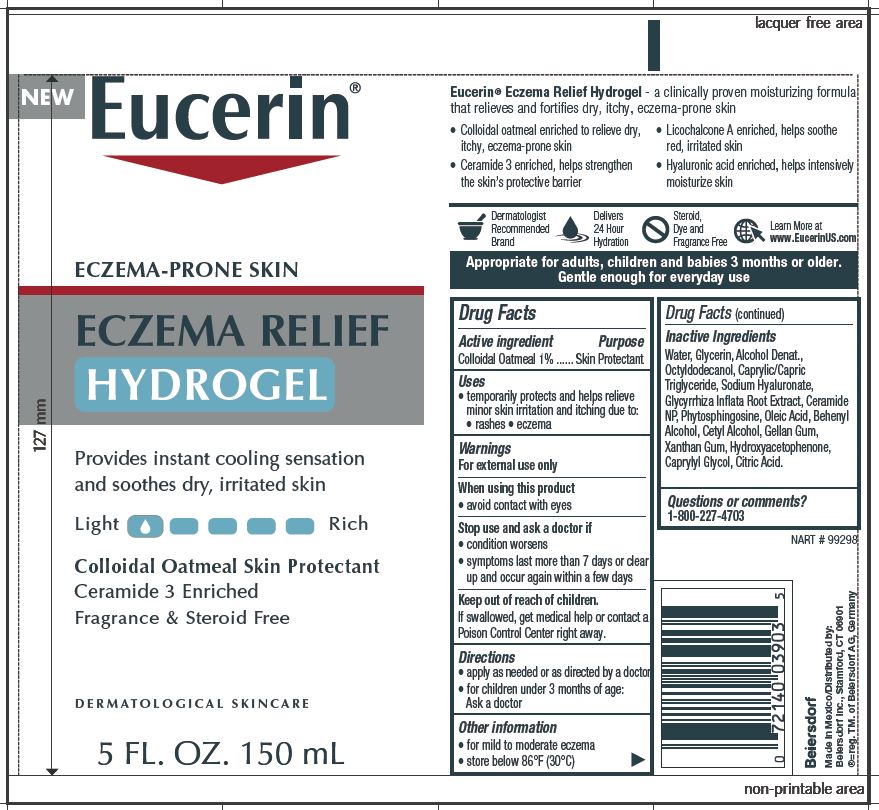
Label: EUCERIN ECZEMA RELIEF HYDROGEL- oatmeal gel
- NDC Code(s): 10356-390-03, 10356-390-35
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 15, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- QUESTIONS
-
INACTIVE INGREDIENT
Inactive Ingredients
Water, Glycerin, Alcohol Denat.,
Octyldodecanol, Caprylic/Capric
Triglyceride, Sodium Hyaluronate,
Glycyrrhiza Inflata Root Extract, Ceramide
NP, Phytosphingosine, Oleic Acid, Behenyl
Alcohol, Cetyl Alcohol, Gellan Gum,
Xanthan Gum, Hydroxyacetophenone,
Caprylyl Glycol, Citric Acid. -
PRINCIPAL DISPLAY PANEL
Eucerin
Eczema Relief Hydrogel
Eczema Prone Skin
Provides instant cooling sensation
and soothes dry, irritated skinColloidal Oatmeal Skin Protectant
Ceramide 3 Enriched
Fragrance & Steroid FreeDERMATOLOGICAL SKINCARE
Eucerin Baby
Eczema Relief Hydrogel
Eczema Prone Skin
Provides instant cooling sensation
and soothes dry, irritated skinColloidal Oatmeal Skin Protectant
Ceramide 3 Enriched
Fragrance & Steroid FreeDERMATOLOGICAL SKINCARE

-
INGREDIENTS AND APPEARANCE
EUCERIN ECZEMA RELIEF HYDROGEL
oatmeal gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10356-390 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) CETYL ALCOHOL (UNII: 936JST6JCN) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) WATER (UNII: 059QF0KO0R) OLEIC ACID (UNII: 2UMI9U37CP) OCTYLDODECANOL (UNII: 461N1O614Y) GLYCYRRHIZA INFLATA ROOT (UNII: 1MV1Z7MKVQ) GLYCERIN (UNII: PDC6A3C0OX) GELLAN GUM (LOW ACYL) (UNII: 7593U09I4D) XANTHAN GUM (UNII: TTV12P4NEE) DOCOSANOL (UNII: 9G1OE216XY) HYALURONATE SODIUM (UNII: YSE9PPT4TH) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CERAMIDE NP (UNII: 4370DF050B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10356-390-35 150 mL in 1 TUBE; Type 0: Not a Combination Product 06/17/2024 2 NDC:10356-390-03 14 mL in 1 TUBE; Type 0: Not a Combination Product 06/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/17/2024 Labeler - Beiersdorf Inc (001177906)