Label: MUCINEX CHILDRENS MIGHTY CHEWS COUGH NIGHTTIME- dextromethorphan hbr and doxylamine succinate tablet, chewable
- NDC Code(s): 72854-082-16
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 5, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
-
Uses
Uses
■ temporarily relieves cough due to minor throat and bronchial irritation as
may occur with a cold
■ controls cough to help you get to sleep
■ temporarily relieves these symptoms due to hay fever or other upper
respiratory allergies:
■ runny nose ■ sneezing
■ itching of the nose or throat ■ itchy, watery eyes -
WARNINGS
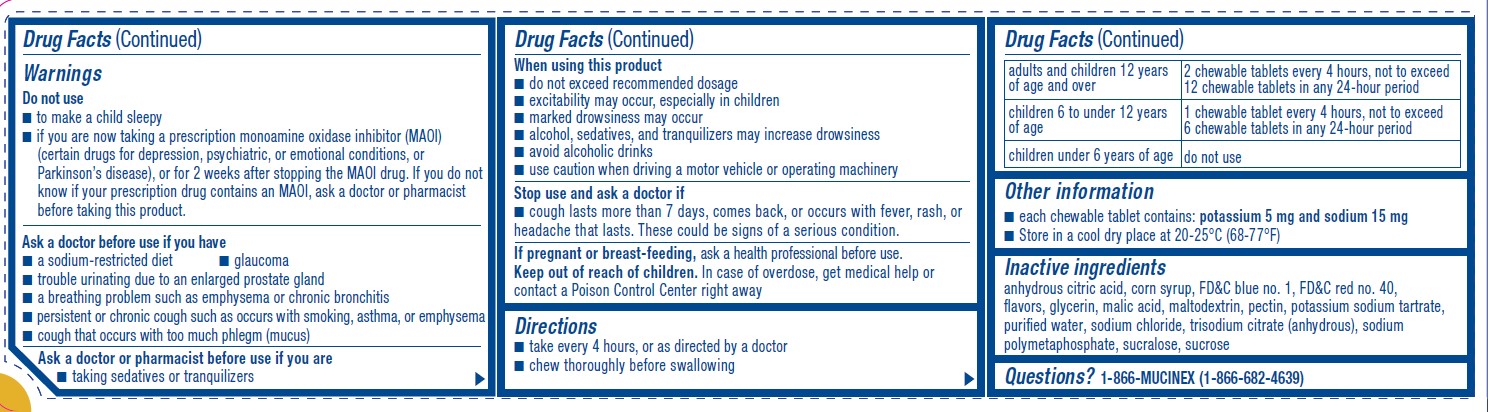
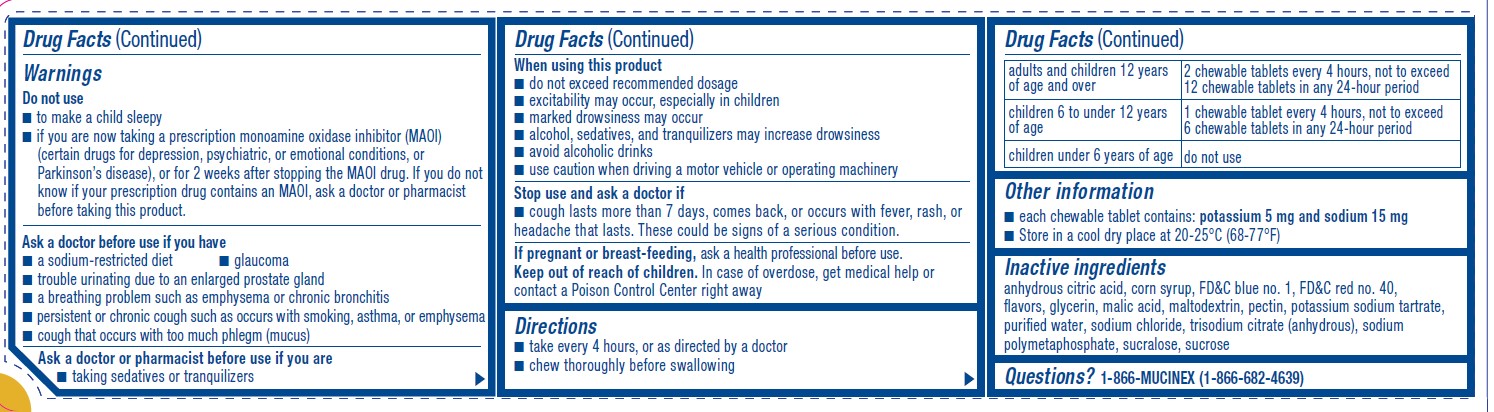
Warnings
Do not use
■ to make a child sleepy
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI)
(certain drugs for depression, psychiatric, or emotional conditions, or
Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not
know if your prescription drug contains an MAOI, ask a doctor or pharmacist
before taking this product.Ask a doctor before use if you have
■ a sodium-restricted diet ■ glaucoma
■ trouble urinating due to an enlarged prostate gland
■ a breathing problem such as emphysema or chronic bronchitis
■ persistent or chronic cough such as occurs with smoking, asthma, or emphysema
■ cough that occurs with too much phlegm (mucus)Ask a doctor or pharmacist before use if you are
■ taking sedatives or tranquilizersWhen using this product
■ do not exceed recommended dosage
■ excitability may occur, especially in children
■ marked drowsiness may occur
■ alcohol, sedatives, and tranquilizers may increase drowsiness
■ avoid alcoholic drinks
■ use caution when driving a motor vehicle or operating machineryStop use and ask a doctor if
■ cough lasts more than 7 days, comes back, or occurs with fever, rash, or
headache that lasts. These could be signs of a serious condition. - KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
■ take every 4 hours, or as directed by a doctor
■ chew thoroughly before swallowingadults and children 12 years
of age and over2 chewable tablets every 4 hours, not to exceed
12 chewable tablets in any 24-hour periodchildren 6 to under 12 years
of age1 chewable tablet every 4 hours, not to exceed
6 chewable tablets in any 24-hour periodchildren under 6 years of age do not use - OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MUCINEX CHILDRENS MIGHTY CHEWS COUGH NIGHTTIME
dextromethorphan hbr and doxylamine succinate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72854-082 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) MALTODEXTRIN (UNII: 7CVR7L4A2D) PECTIN (UNII: 89NA02M4RX) POTASSIUM SODIUM TARTRATE (UNII: QH257BPV3J) WATER (UNII: 059QF0KO0R) MALIC ACID (UNII: 817L1N4CKP) CORN SYRUP (UNII: 9G5L16BK6N) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) SODIUM CHLORIDE (UNII: 451W47IQ8X) ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) SUCROSE (UNII: C151H8M554) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color purple Score no score Shape ROUND Size 23mm Flavor BERRY (Mixed Berry) Imprint Code M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72854-082-16 16 in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 06/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/03/2024 Labeler - RB Health (US) LLC (081049410)