Label: LARTT MOUTHWASH- sodium fluoride liquid
- NDC Code(s): 61284-0014-1, 61284-0014-2
- Packager: ECOWORLDPHARM CO.,LTD
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 5, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
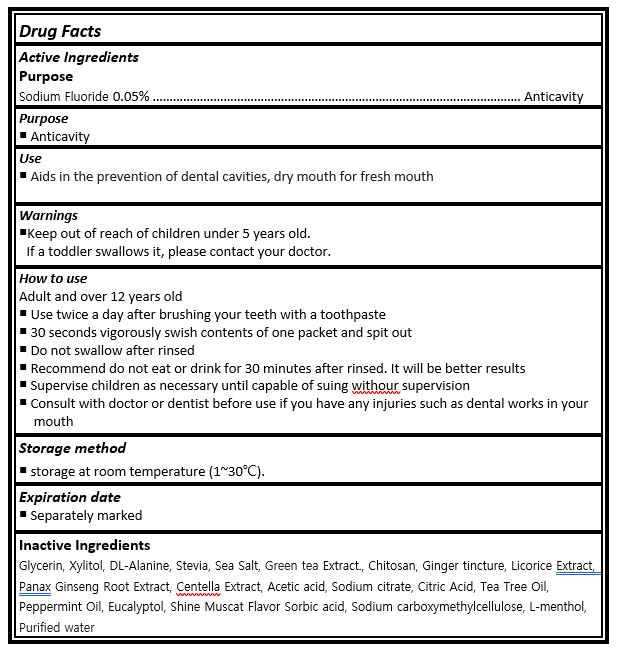
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Glycerin, Xylitol, DL-Alanine, Stevia, Sea Salt, Green tea Extract., Chitosan, Ginger tincture, Licorice Extract, Panax Ginseng Root Extract, Centella Extract, Acetic acid, Sodium citrate, Citric Acid, Tea Tree Oil, Peppermint Oil, Eucalyptol, Shine Muscat Flavor Sorbic acid, Sodium carboxymethylcellulose, L-menthol, Purified water
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Adult and over 12 years old
¡ Use twice a day after brushing your teeth with a toothpaste
¡ 30 seconds vigorously swish contents of one packet and spit out
¡ Do not swallow after rinsed
¡ Recommend do not eat or drink for 30 minutes after rinsed. It will be better results
¡ Supervise children as necessary until capable of suing withour supervision
¡ Consult with doctor or dentist before use if you have any injuries such as dental works in your mouth
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LARTT MOUTHWASH
sodium fluoride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61284-0014 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) PANAX GINSENG ROOT OIL (UNII: P9T4K47OM0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61284-0014-2 30 in 1 PACKAGE 10/05/2023 1 NDC:61284-0014-1 13 mL in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 10/05/2023 Labeler - ECOWORLDPHARM CO.,LTD (688735061) Registrant - ECOWORLDPHARM CO.,LTD (688735061) Establishment Name Address ID/FEI Business Operations ECOWORLDPHARM CO.,LTD 688735061 manufacture(61284-0014)