Label: CAUDALIE DAILY MINERAL SUNSCREEN BROAD SPECTRUM SPF 50- zinc oxide lotion
- NDC Code(s): 76296-060-30, 76296-060-39, 76296-060-50
- Packager: Caudalie USA, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
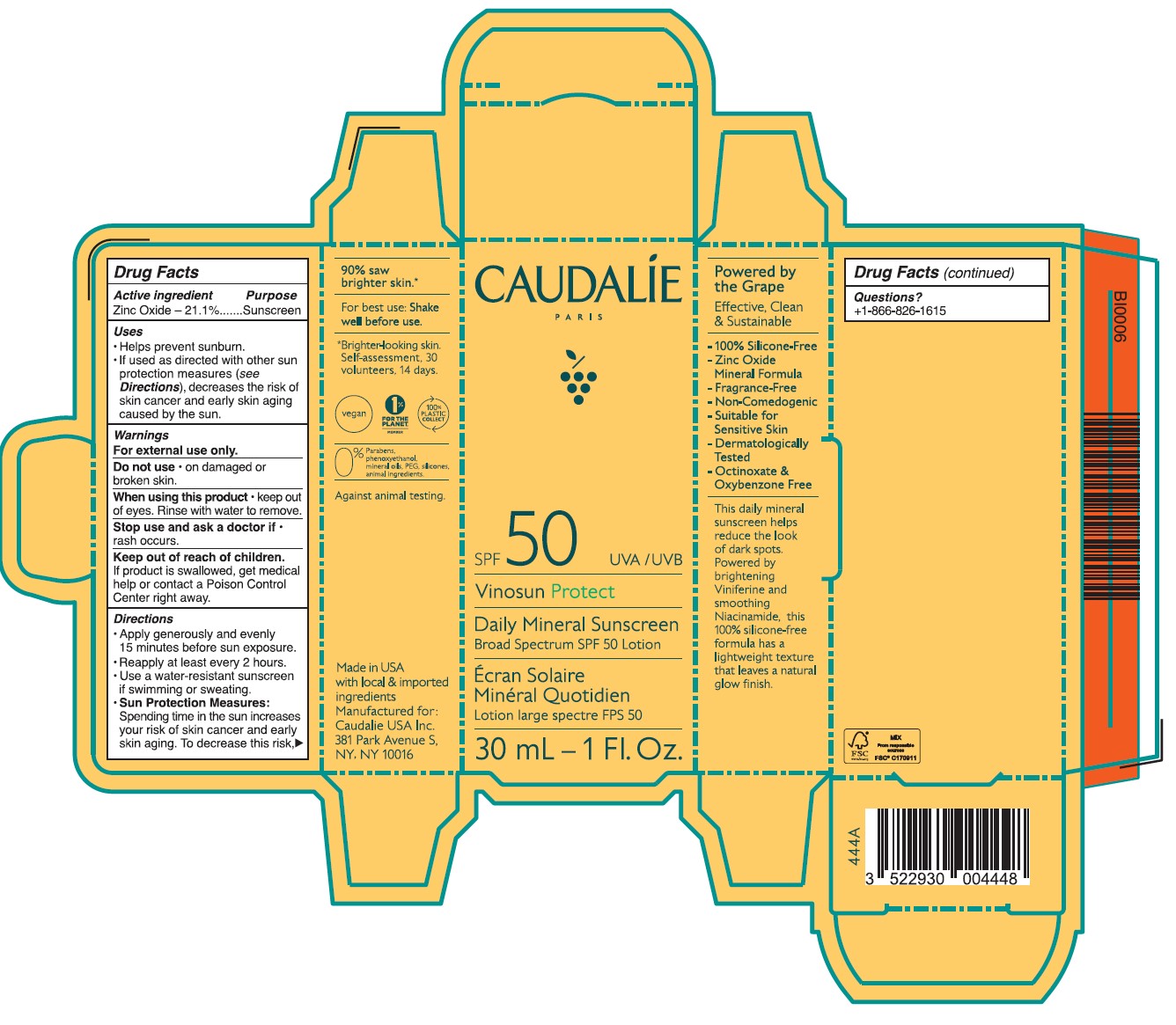
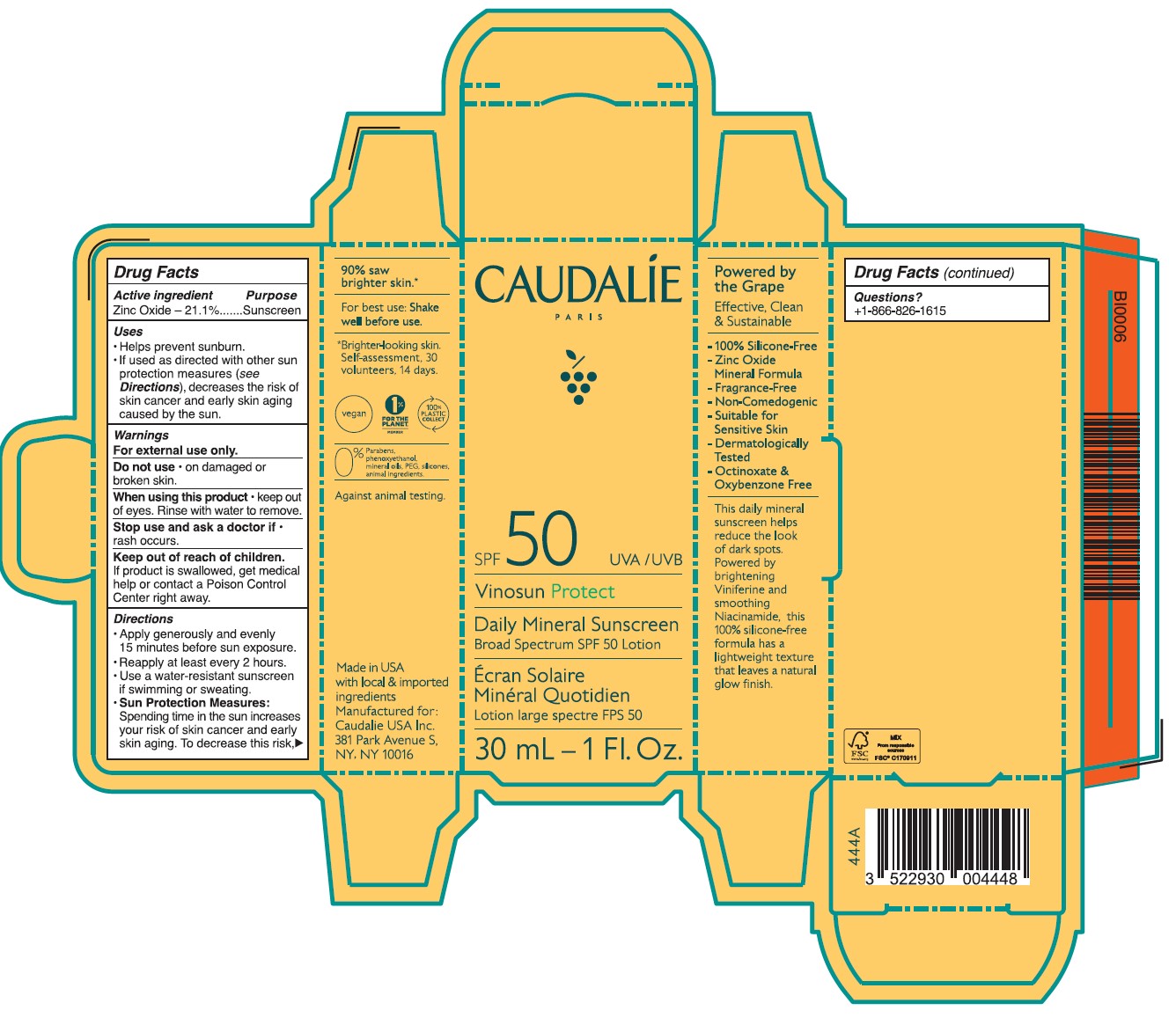
- Drug Facts
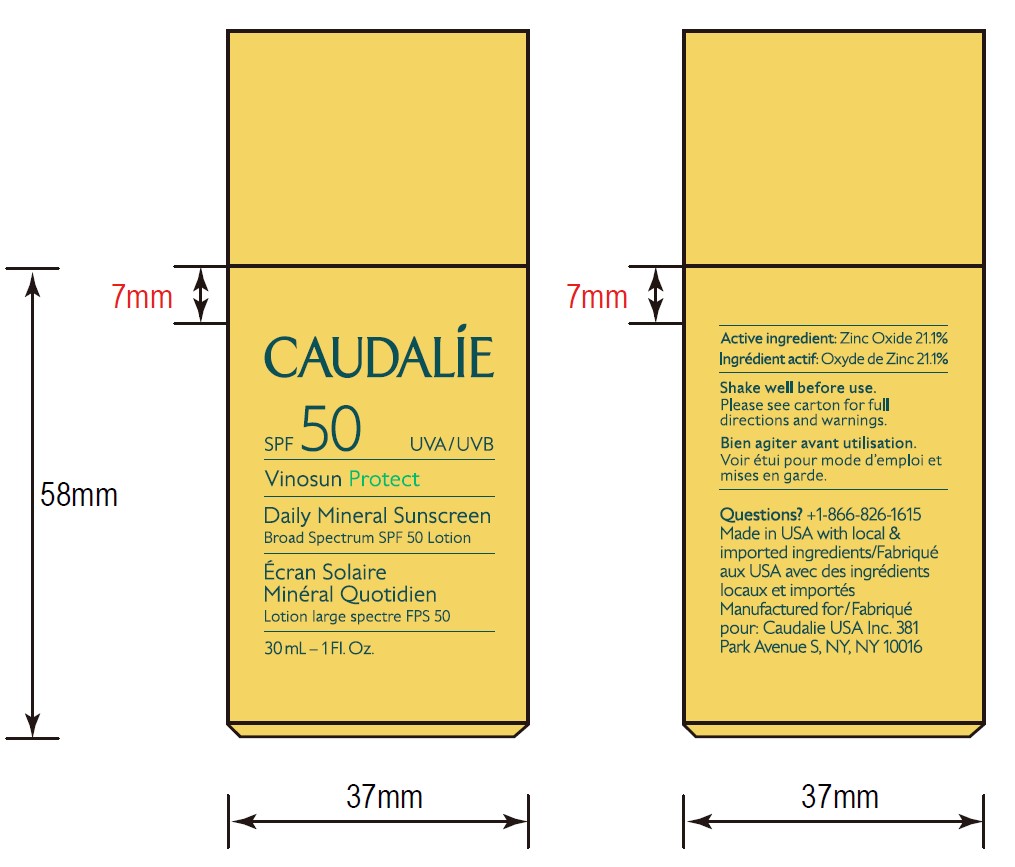
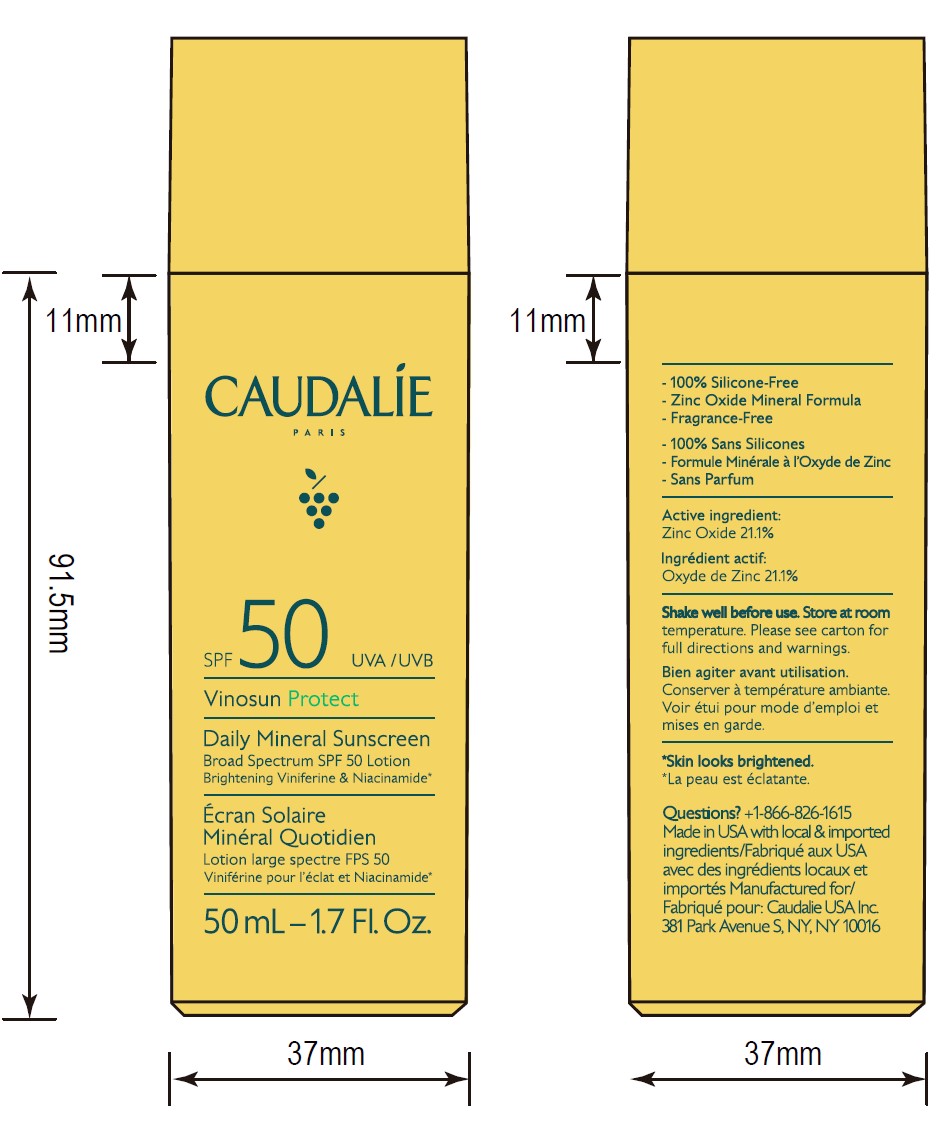
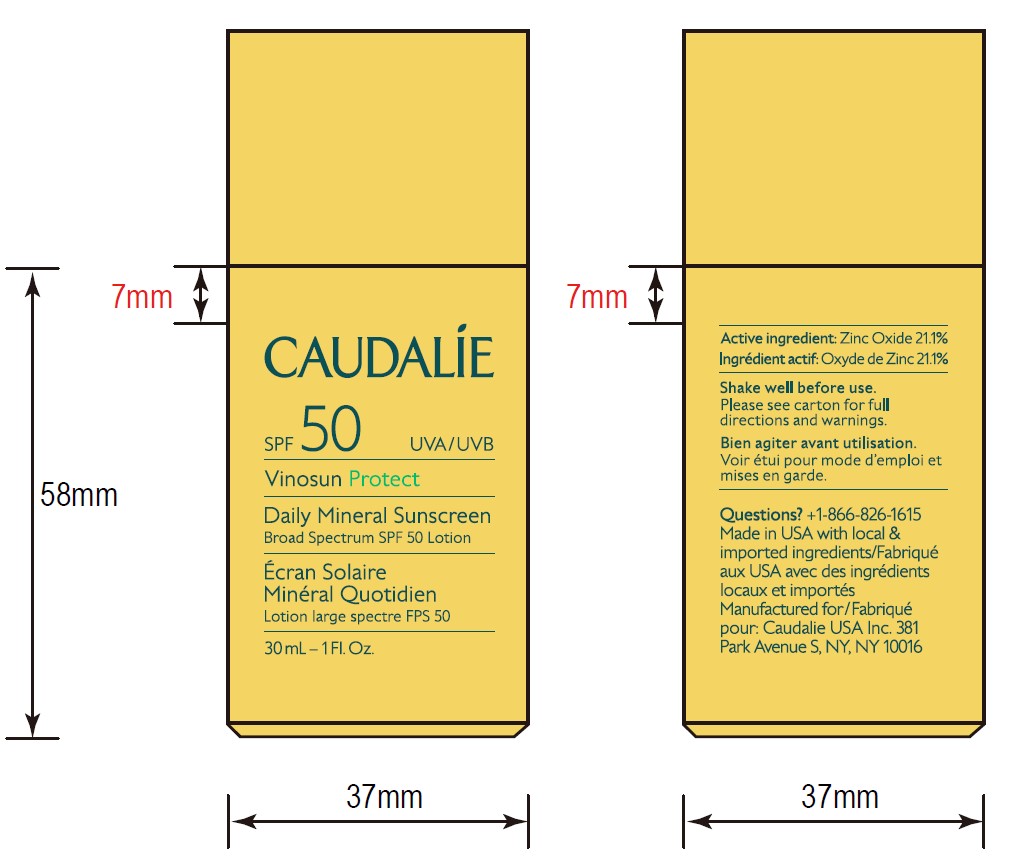
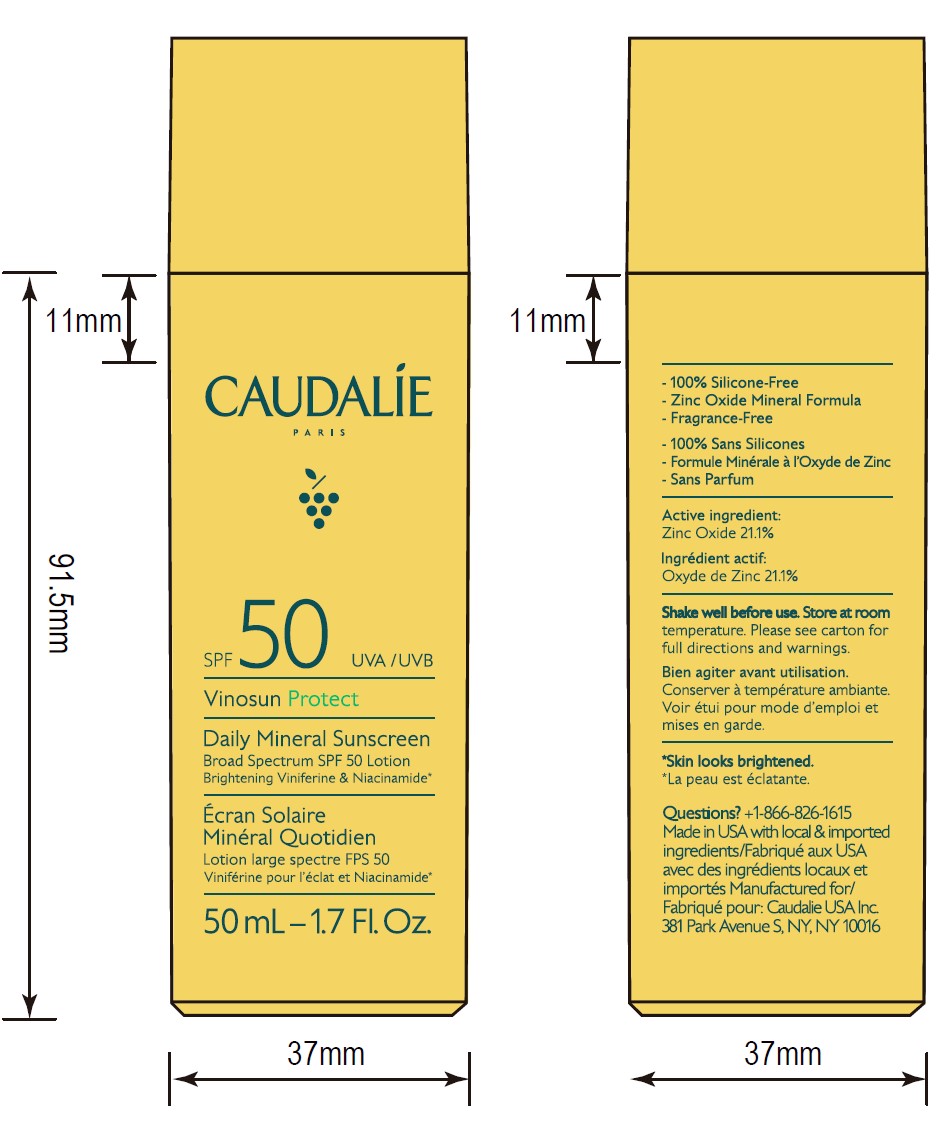
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- Warnings
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
• Apply generously and evenly 15 minutes before sun exposure.
• Reapply at least every 2 hours • Use a water-resistant sunscreen if swimming or sweating.
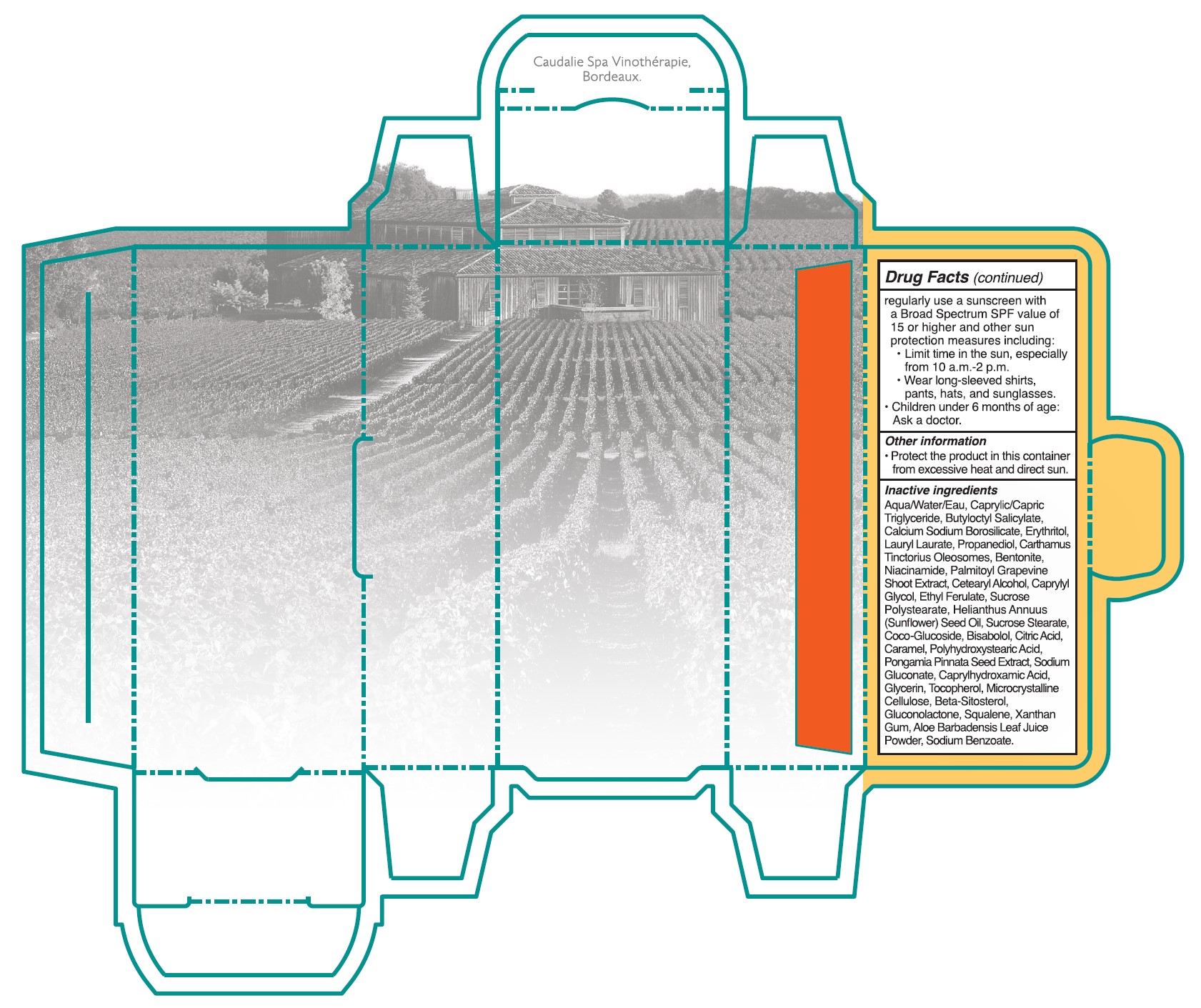
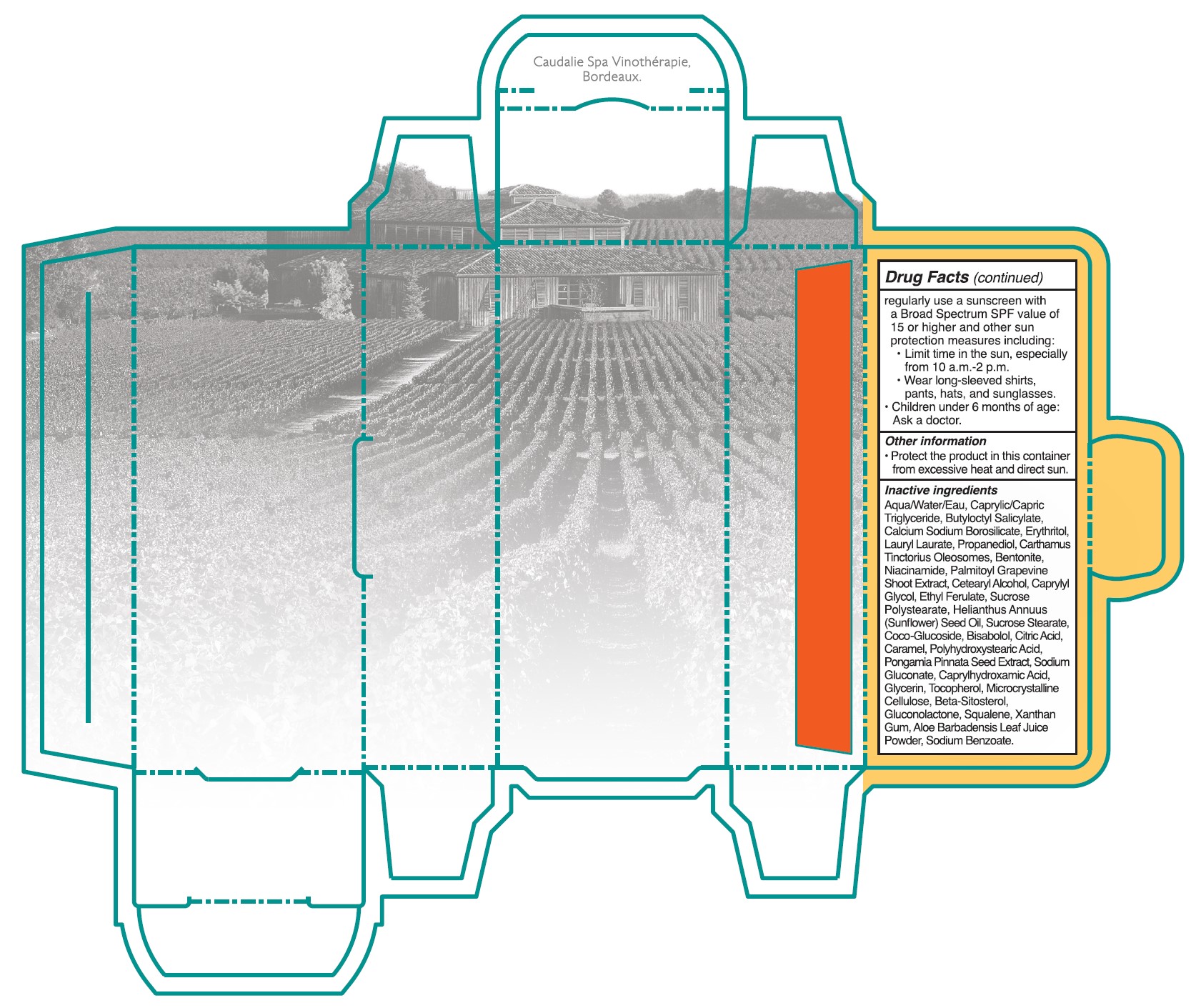
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regulalry use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
•Limit time in the sun, especially from 10 a.m. - 2 p.m.
•Wear long-sleeved shirts, pants, hats, and singlasses.
•Children under 6 months of age: Ask a doctor.
- Other information
-
Inactive ingredients
Aqua/Water/Eau, Caprylic/Capric Triglyceride, Butyloctyl Salicylate, Calcium Sodium Borosilicate, Erythritol, Lauryl Laurate, Propanediol, Carthamus Tinctorius Oleosomes, Bentonite, Niacinamide, Palmitoyl Grapevine Shoot Extract, Cetearyl Alcohol, Caprylyl Glycol, Ethyl Ferulate, Sucrose Polystearate, Helianthus Annuus (Sunflower) Seed Oil,Sucrose Stearate, Coco-Glucoside, Bisabolol, Citric Acid, Caramel, Polyhydroxystearic Acid, Pongamia Pinnata Seed Extract, Sodium Gluconate, Caprylhydroxamic Acid, Glycerin, Tocopherol, Microcrystallnine Cellulose, Beta-Sitosterol, Gluconolactone, Squalene, Xanthan Gum, Aloe Barbadensis Leaf Juice Powder, Sodium Benzoate.
- Questions?
- 30 mL Pack
- 50 mL Pack
- Co-pack
-
INGREDIENTS AND APPEARANCE
CAUDALIE DAILY MINERAL SUNSCREEN BROAD SPECTRUM SPF 50
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76296-060 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 13.1875 g in 62.5 g Inactive Ingredients Ingredient Name Strength PROPANEDIOL (UNII: 5965N8W85T) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) GLYCERIN (UNII: PDC6A3C0OX) SODIUM GLUCONATE (UNII: R6Q3791S76) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) LEVOMENOL (UNII: 24WE03BX2T) .BETA.-SITOSTEROL (UNII: S347WMO6M4) GLUCONOLACTONE (UNII: WQ29KQ9POT) SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CARAMEL (UNII: T9D99G2B1R) PONGAMIA PINNATA SEED (UNII: C2BRV53B1V) XANTHAN GUM (UNII: TTV12P4NEE) BOROSILICATE GLASS (UNII: BOJ6T9AR90) TOCOPHEROL (UNII: R0ZB2556P8) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALOE VERA LEAF (UNII: ZY81Z83H0X) SUNFLOWER OIL (UNII: 3W1JG795YI) ERYTHRITOL (UNII: RA96B954X6) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) LAURYL LAURATE (UNII: GPW77G0937) BENTONITE (UNII: A3N5ZCN45C) ETHYL FERULATE (UNII: 5B8915UELW) COCO GLUCOSIDE (UNII: ICS790225B) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SUCROSE STEARATE (UNII: 274KW0O50M) SQUALENE (UNII: 7QWM220FJH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76296-060-30 1 in 1 CARTON 05/20/2024 1 37.5 g in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:76296-060-50 1 in 1 CARTON 05/20/2024 2 62.5 g in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:76296-060-39 1 in 1 CARTON 05/20/2024 3 37.5 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/20/2024 Labeler - Caudalie USA, Inc. (100064349)