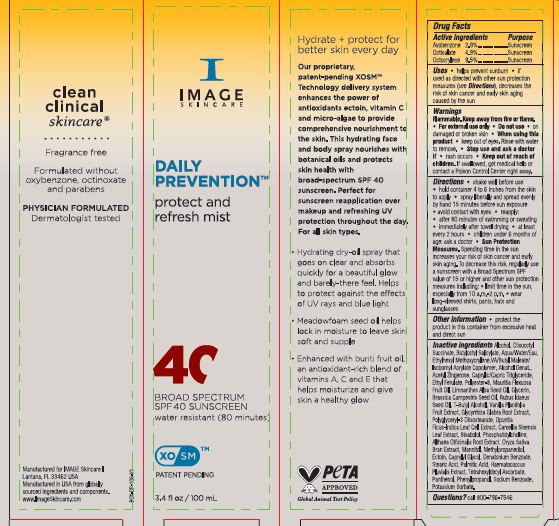
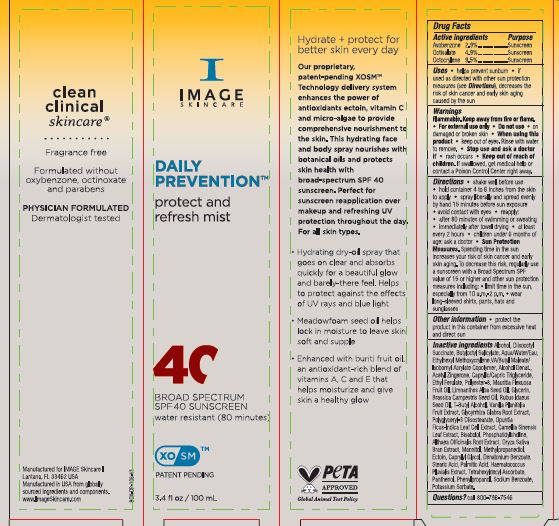
Label: DAILY PREVENTION PROTECT AND REFRESH MIST SPF 40- avobenzone, octisalate and octocrylene spray
- NDC Code(s): 62742-4257-1, 62742-4257-2
- Packager: Allure Labs
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 21, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions • shake well before use • hold container 4 to 6 inches from the skin to apply • spray liberally and spread evenly by hand 15 minutes before sun exposure • avoid contact with eyes • reapply: • after 80 minutes of swimming or sweating • immediately after towel drying • at least every 2 hours • children under 6 months of age: ask a doctor • Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m.-2 p.m. • wear long-sleeved shirts, pants, hats and sunglasses
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients: Alcohol, Diisooctyl Succinate, Butyloctyl Salicylate, Aqua/water/Eau, Ethylhexyl Methoxycrylene, VA/Butyl Maleate/ Isobornyl Acrylate Copolymer, Alcohol Denat., Acetyl Zingerone, Caprylie/Capric Triglyceride, Ethyl Ferulate, Polyester-8, Mauritia Flexuosa Fruit Oil, Limnanthes Alba Seed Oil, Glycerin, Brassica Campestris Seed Oil, Rubus Idaeus Seed Oil, T-Butyl Alcohol, Vanilla Planifolia Fruit Extract, Glycyrrhiza Glabra Root Extract, Polyglyceryl-3 Diisostearate, Opuntia Ficus-Indica Leaf Cell Extract, Camellia Sinensis Leaf Extract, Bisabolol, Phosphatidylcholine, Althaea Officinalis Root Extract, Oryza Sativa Bran Extract, Mannitol, Methylpropanediol, Ectoin, Caprylyl Glycol, Denatonium Benzoate, Stearic Acid, Palmitic Acid, Haematococcus Pluvialis Extract, Tetrahexyldecyl Ascorbate, Panthenol, Phenylpropanol, Sodium Benzoate, Potassium Sorbate.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAILY PREVENTION PROTECT AND REFRESH MIST SPF 40
avobenzone, octisalate and octocrylene sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4257 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9.5 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.9 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.9 g in 100 g Inactive Ingredients Ingredient Name Strength OPUNTIA FICUS-INDICA LEAF (UNII: 5VM709H93V) GREEN TEA LEAF (UNII: W2ZU1RY8B0) DIISOBUTYL SUCCINATE (UNII: 1241X4J800) ISOBORNYL ACRYLATE (UNII: IX0PRH184P) ACETYL ZINGERONE (UNII: V9D92S9YE5) STEARIC ACID (UNII: 4ELV7Z65AP) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ETHYL FERULATE (UNII: 5B8915UELW) RUBUS IDAEUS SEED (UNII: M3CL7US2ZG) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) PANTHENOL (UNII: WV9CM0O67Z) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) LEVOMENOL (UNII: 24WE03BX2T) ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) METHYLPROPANEDIOL (UNII: N8F53B3R4R) ECTOINE (UNII: 7GXZ3858RY) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) HAEMATOCOCCUS PLUVIALIS (UNII: 31T0FF0472) VANILLA BEAN (UNII: Q74T35078H) RICE BRAN (UNII: R60QEP13IC) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PALMITIC ACID (UNII: 2V16EO95H1) SODIUM BENZOATE (UNII: OJ245FE5EU) WATER (UNII: 059QF0KO0R) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) DIBUTYL MALEATE (UNII: 4X371TMK9K) MAURITIA FLEXUOSA FRUIT OIL (UNII: 48H19MS04L) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) GLYCERIN (UNII: PDC6A3C0OX) BRASSICA RAPA SUBSP. RAPA SEED (UNII: 728X944L6M) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) ALCOHOL (UNII: 3K9958V90M) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) PHENYLPROPANOL (UNII: 0F897O3O4M) PHOSPHATIDYLCHOLINE TRANSLOCATOR ABCB4 (UNII: 9EI49ZU76O) MANNITOL (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4257-2 1 in 1 CARTON 03/21/2024 1 NDC:62742-4257-1 100 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/21/2024 Labeler - Allure Labs (926831603) Registrant - Allure Labs (926831603) Establishment Name Address ID/FEI Business Operations Allure Labs 926831603 manufacture(62742-4257)