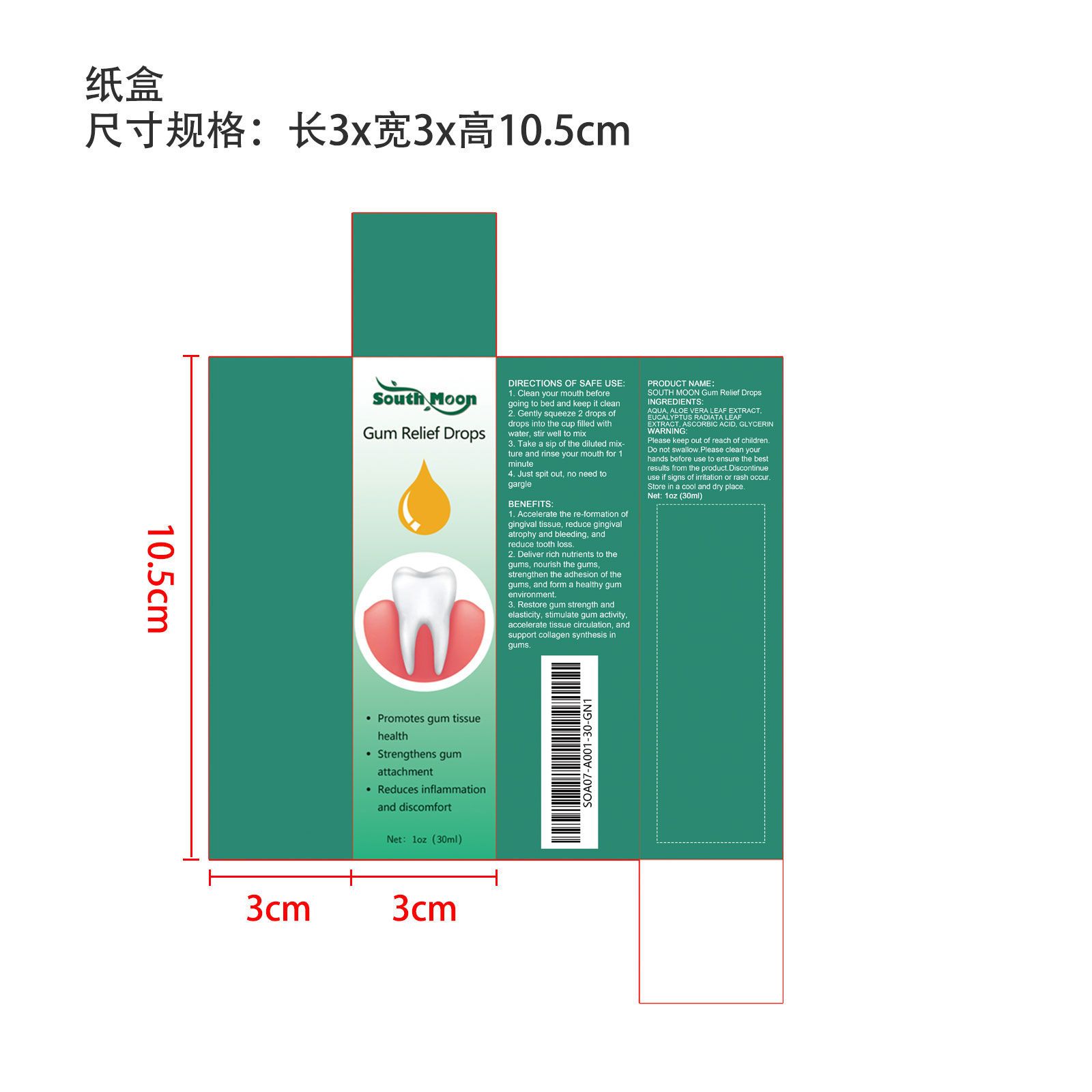
Label: GUM RELIEF DROPS liquid
- NDC Code(s): 84067-142-01
- Packager: Shantou Youjia E-Commerce Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SOUTH MOON Gum Relief Drops
-
INGREDIENTS AND APPEARANCE
GUM RELIEF DROPS
gum relief drops liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84067-142 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUCALYPTUS RADIATA LEAF (UNII: SKT7DM564Q) (EUCALYPTUS RADIATA LEAF - UNII:SKT7DM564Q) EUCALYPTUS RADIATA LEAF 6 g in 30 g ALOE VERA LEAF (UNII: ZY81Z83H0X) (ALOE VERA LEAF - UNII:ZY81Z83H0X) ALOE VERA LEAF 7.5 g in 30 g ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 4.5 g in 30 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 3 g in 30 g WATER (UNII: 059QF0KO0R) 9 mL in 30 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84067-142-01 30 g in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 02/01/2024 12/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 02/01/2024 12/31/2024 Labeler - Shantou Youjia E-Commerce Co., Ltd. (711173127) Establishment Name Address ID/FEI Business Operations Shantou Youjia E-Commerce Co., Ltd. 711173127 label(84067-142)