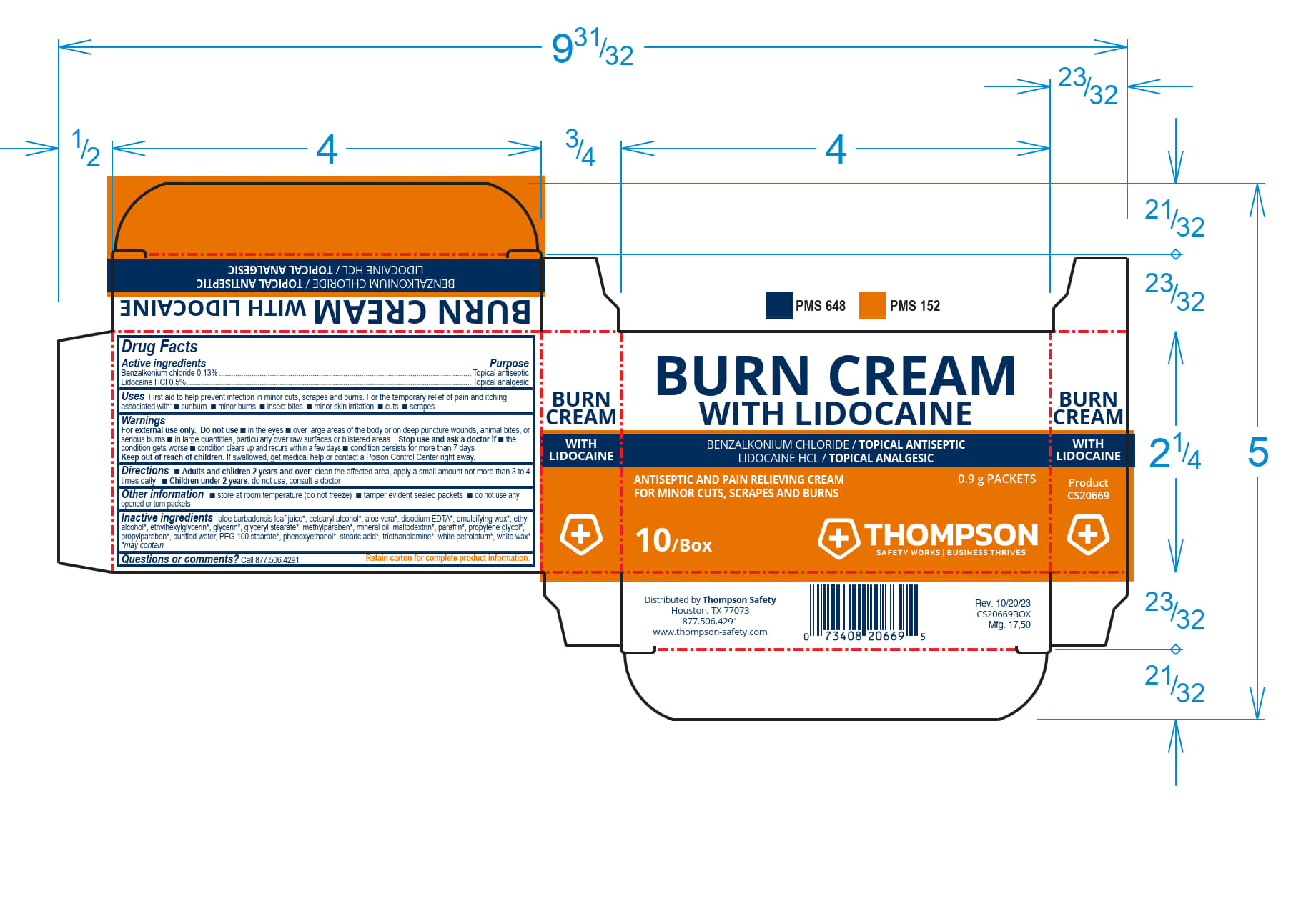
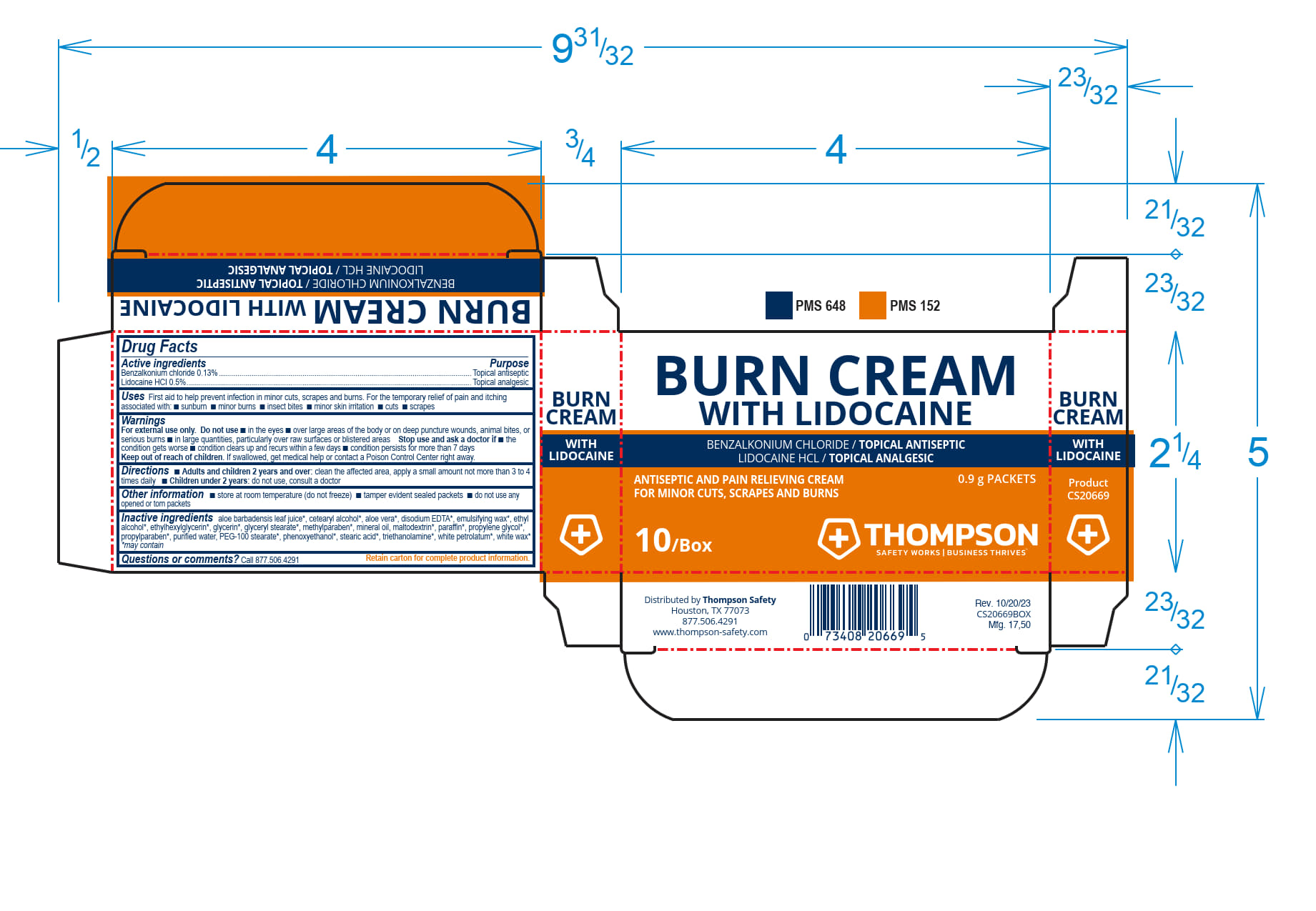
Label: THOMPSON BURN CREAM WITH LIDOCAIN- benzalkonium chloride, lidocaine cream
THOMPSON BURN CREAM WITH LIDOCAINE- benzalkonium chloride, lidocaine cream
- NDC Code(s): 73408-060-12, 73408-160-12, 73408-940-73, 73408-985-73
- Packager: Thompson Safety
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 13, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients
aloe barbadensis leaf juice*, cetearyl alcohol*, aloe vera*, disodium EDTA*, emulsifying wax*, ethyl alcohol*, ethylhexlglycerin*, glycerin*, glyceryl stearate*, methylparaben*, mineral oil, maltodextrin*, paraffin*, propylene glycol*, propylparaben*, purifed water*, PEG-100 stearate*, phenoxyethanol*, stearic acid*, triethanolamine*, white petrolatum*, white wa*x
*may contain
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THOMPSON BURN CREAM WITH LIDOCAIN
benzalkonium chloride, lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73408-060 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETEARYL ALCOHOL (UNII: 2DMT128M1S) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) MALTODEXTRIN (UNII: 7CVR7L4A2D) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) MINERAL OIL (UNII: T5L8T28FGP) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TRIETHANOLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73408-060-12 10 in 1 BOX 11/30/2023 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 11/30/2023 THOMPSON BURN CREAM WITH LIDOCAINE
benzalkonium chloride, lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73408-160 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) METHYLPARABEN (UNII: A2I8C7HI9T) WHITE WAX (UNII: 7G1J5DA97F) ALCOHOL (UNII: 3K9958V90M) PARAFFIN (UNII: I9O0E3H2ZE) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73408-160-12 10 in 1 BOX 11/30/2023 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 11/30/2023 THOMPSON BURN CREAM WITH LIDOCAINE
benzalkonium chloride, lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73408-985 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) CETEARYL ALCOHOL (UNII: 2DMT128M1S) ALOE VERA LEAF (UNII: ZY81Z83H0X) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) PEG-100 STEARATE (UNII: YD01N1999R) MINERAL OIL (UNII: T5L8T28FGP) MALTODEXTRIN (UNII: 7CVR7L4A2D) TRIETHANOLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73408-985-73 25 in 1 BOX 02/16/2024 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/16/2024 THOMPSON BURN CREAM WITH LIDOCAINE
benzalkonium chloride, lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73408-940 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PROPYLPARABEN (UNII: Z8IX2SC1OH) PETROLATUM (UNII: 4T6H12BN9U) WHITE WAX (UNII: 7G1J5DA97F) MINERAL OIL (UNII: T5L8T28FGP) METHYLPARABEN (UNII: A2I8C7HI9T) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) PARAFFIN (UNII: I9O0E3H2ZE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73408-940-73 25 in 1 BOX 02/16/2024 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/16/2024 Labeler - Thompson Safety (080998015) Registrant - Unifirst First Aid Corporation (832947092) Establishment Name Address ID/FEI Business Operations Prestige Packaging 080667761 pack(73408-940, 73408-985, 73408-060, 73408-160) Establishment Name Address ID/FEI Business Operations Medique Products 086911794 pack(73408-985, 73408-940, 73408-060, 73408-160)