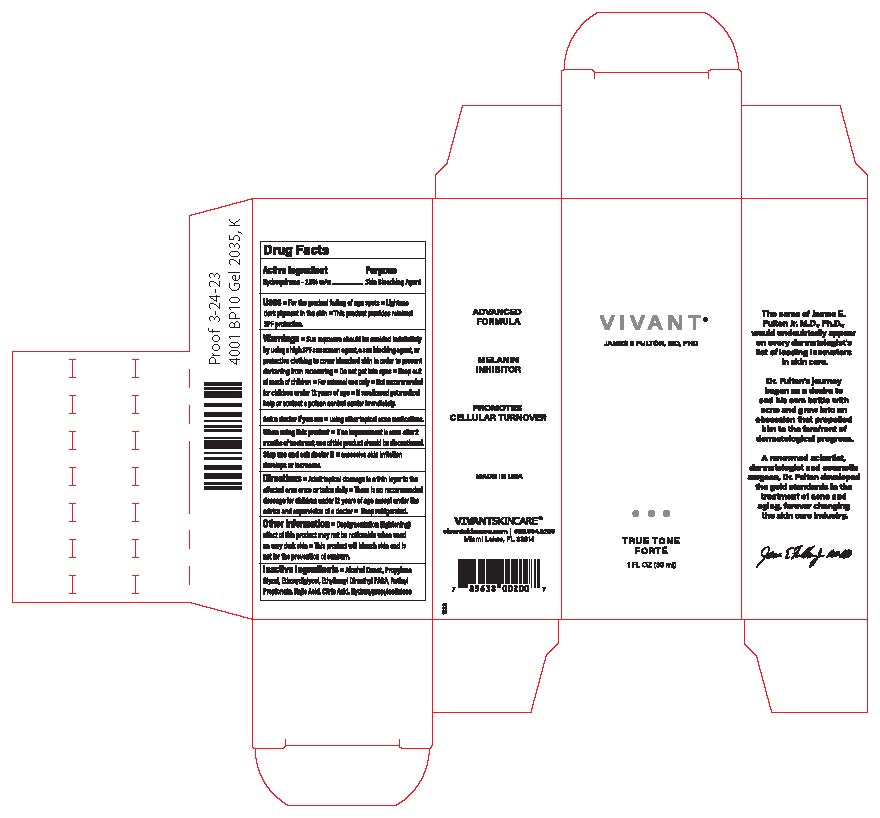
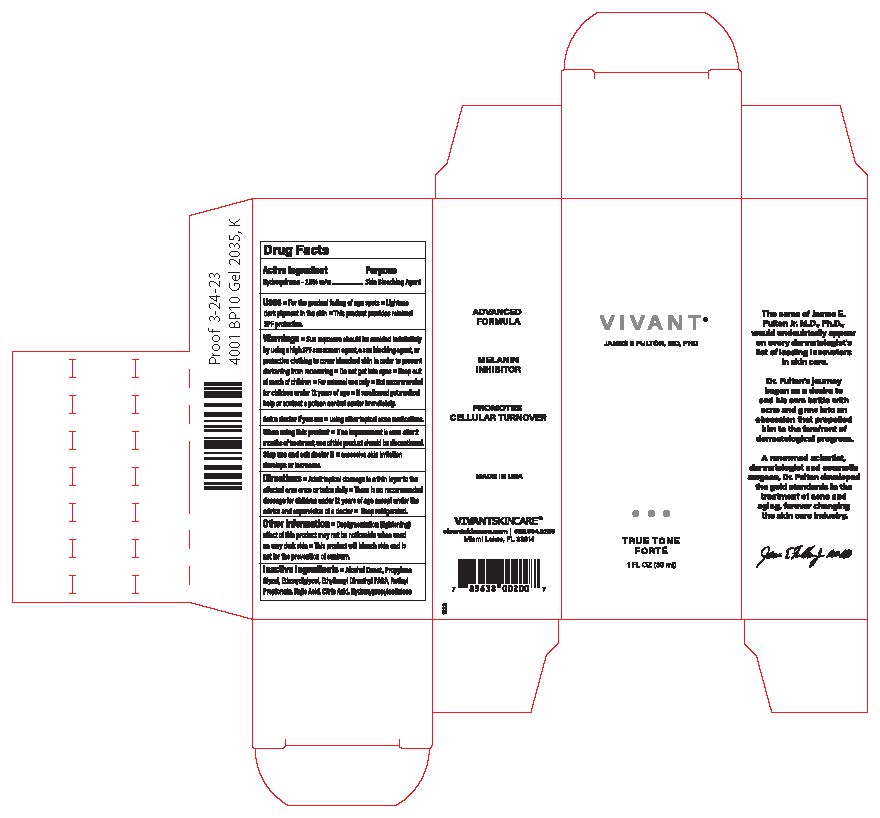
Label: VIVANT TRUE TONE FORTE- hydroquinone gel
- NDC Code(s): 63750-001-01
- Packager: Vivant Pharmaceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 6, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Indication
- Uses
-
Warnings
Warnings ■Sun exposure should be avoided indefinitely by using a high SPF sunscreen agent, a sunblocking agent, or protective clothing to cover bleached skin in order to prevent darkening from reoccurring ■ Do not get into eyes ■Keep out of reach of
children ■For external use only ■Not recommended for children under 12 years of age ■ If swallowed get medical help or contact a poison control center immediately.
- Ask a doctor if you are:
- Directions
- Inactive Ingredients
- When using this product
- Stop use and ask the doctor if
- Warnings
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
VIVANT TRUE TONE FORTE
hydroquinone gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63750-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROQUINONE (UNII: XV74C1N1AE) (HYDROQUINONE - UNII:XV74C1N1AE) HYDROQUINONE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) PADIMATE O (UNII: Z11006CMUZ) KOJIC ACID (UNII: 6K23F1TT52) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) RETINYL PROPIONATE (UNII: 32JK994WMC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE (11% HYDROXYPROPYL; 140000 MW) (UNII: 599BTP6K9F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63750-001-01 1 in 1 BOX 02/01/2024 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2024 Labeler - Vivant Pharmaceuticals, LLC (782696814) Establishment Name Address ID/FEI Business Operations Vivant Pharmaceuticals, LLC 782696814 manufacture(63750-001)