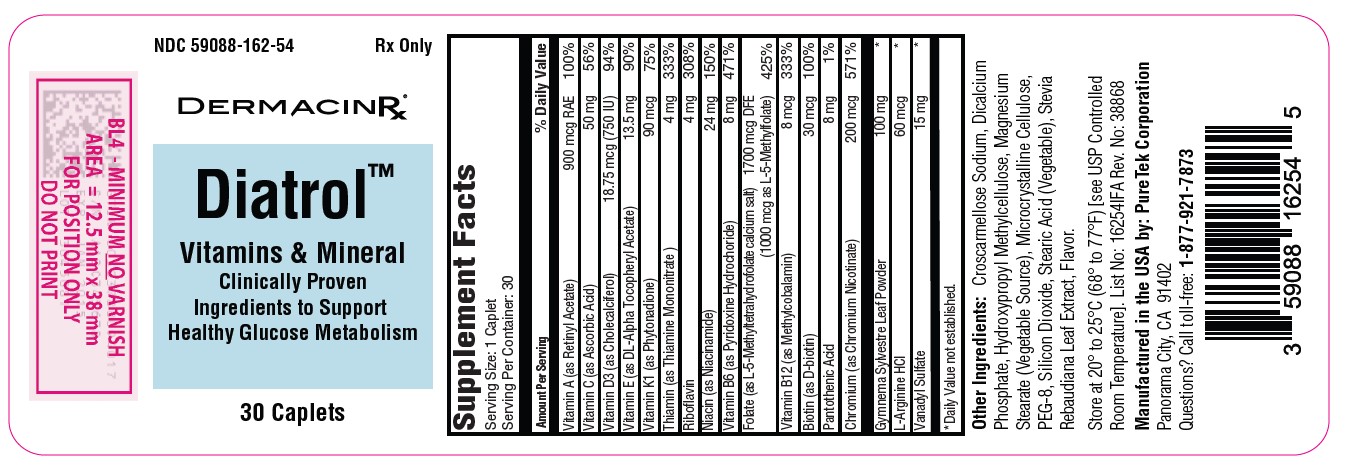
Label: DIATROL- vitamins and mineral capsule
- NDC Code(s): 59088-162-54
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Each Caplet Contains:
Vitamin A (as Retinyl Acetate).................................... 900 mcg RAE
Vitamin C (as Ascorbic Acid)................................................. 50 mg
Vitamin D3 (as Cholecalciferol)....................................... 18.75 mcg
Vitamin E (as DL-Alpha Tocopheryl Acetate)...................... 13.5 mg
Vitamin K1 (as Phytonadione)............................................. 90 mcg
Thiamin (as Thiamine Mononitrate)......................................... 4 mg
Riboflavin ................................................................................. 4 mg
Niacin (as Niacinamide)......................................................... 24 mg
Vitamin B6 (as Pyridoxine Hydrochloride)............................... 8 mg
Folate (as L-5-Methyltetrahydrofolate calcium salt)…1700 mcg DFE
(1000 mcg as L-5-Methylfolate)
Vitamin B12 (as Methylcobalamin)........................................ 8 mcg
Biotin (as D-biotin)............................................................... 30 mcg
Pantothenic Acid ...................................................................... 8 mg
Chromium (as Chromium Nicotinate)............................... 200 mcg
Gymnema Sylvestre Leaf Powder........................................ 100 mg
L-Arginine HCl...................................................................... 60 mcg
Vanadyl Sulfate....................................................................... 15 mg
- Other Ingredients:
-
INDICATIONS AND USAGE
Diatrol™ is indicated to provide significant amounts of essential vitamins and mineral. This comprehensive nutrient profile helps prevent nutritional deficiencies of these vitamins and minerals, ensuring that the specific dietary needs are met to support overall health, energy, and vitality. The product is specially formulated to target common vitamin and mineral gaps, thus promoting optimal health, immune function, bone strength, and metabolic balance. It is intended to be used under the guidance of a licensed healthcare practitioner to ensure that any potential for nutritional deficiency is addressed in a manner that supports the individual's overall health and wellbeing.
- WARNING
-
PRECAUTIONS
Folate doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive. There is a potential danger in administering folate to patients with undiagnosed anemia, since folate may obscure the diagnosis of pernicious anemia by alleviating the hematologic manifestations of the disease while allowing the neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. Adequate doses of vitamin B12 may prevent, halt, or improve the neurologic changes caused by pernicious anemia.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
For use on the order of a licensed healthcare practitioner. Call your doctor about side effects. To report side effects, call PureTek Corporation at 1-877-921-7873 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
Adverse Reactions:
Folate: Allergic sensitizations has been reported following both oral and parenteral administration of folate. Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic and idiosyncratic reactions are possible at lower levels.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- Diatrol™
-
INGREDIENTS AND APPEARANCE
DIATROL
vitamins and mineral capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59088-162 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 30 ug VANADYL SULFATE (UNII: 6DU9Y533FA) (VANADIUM - UNII:00J9J9XKDE) VANADYL SULFATE 15 mg CHROMIUM NICOTINATE (UNII: A150AY412V) (CHROMIC CATION - UNII:X1N4508KF1) CHROMIUM NICOTINATE 200 ug GYMNEMA SYLVESTRE LEAF (UNII: 2ZK6ZS8392) (GYMNEMA SYLVESTRE LEAF - UNII:2ZK6ZS8392) GYMNEMA SYLVESTRE LEAF 100 mg ARGININE HYDROCHLORIDE (UNII: F7LTH1E20Y) (ARGININE - UNII:94ZLA3W45F) ARGININE HYDROCHLORIDE 60 ug .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 13.5 mg PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 90 ug VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 900 ug ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 50 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 18.75 ug PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 8 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 4 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 4 mg NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 24 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 1000 ug METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 8 ug PANTOTHENIC ACID (UNII: 19F5HK2737) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 8 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSES (UNII: 3NXW29V3WO) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Product Characteristics Color green (Light Green with Brown Speckles) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-162-54 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/26/2024 Labeler - PureTek Corporation (785961046)