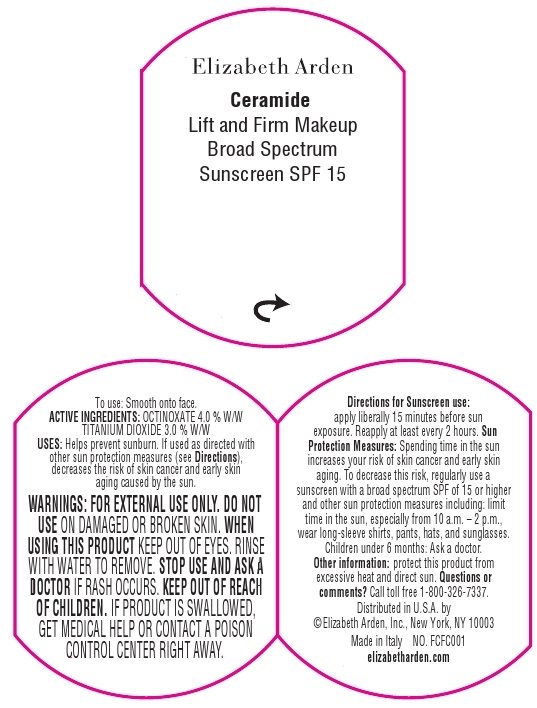
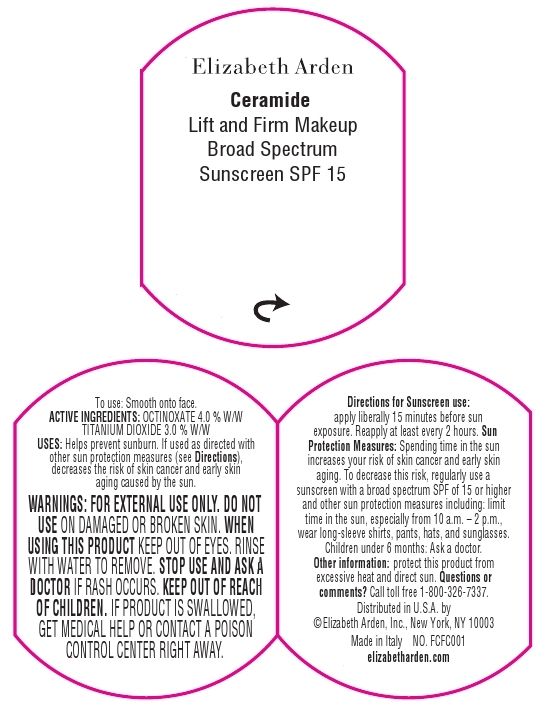
Label: CERAMIDE LIFT AND FIRM MAKEUP BROAD SPECTRUM SUNSCREEN SPF 15 SPICE- octinoxate and titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 67938-0951-1, 67938-0951-2, 67938-0951-3 - Packager: Elizabeth Arden, Inc
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 5, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
- For normal to dry skin.
- Medium to full coverage. Luminous finish.
Makeup goes ultra to smooth, lift, and firm the look of skin. This moisturizing cream foundation with CPT technology, Ceramides, and Advanced Moisture Complex diminished the appearance of fine lines, perfects skin tone, and creates a luminous finish that lasts all day. Provides Broad Spectrum Sunscreen SPF 15 protection.
-
INDICATIONS AND USAGE
To Use: Smooth onto face.
For Sunscreen Use: Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours.
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m. - 2 p.m. Wear long-sleeve shirts, pants, hats, and sunglasses.
- WARNINGS
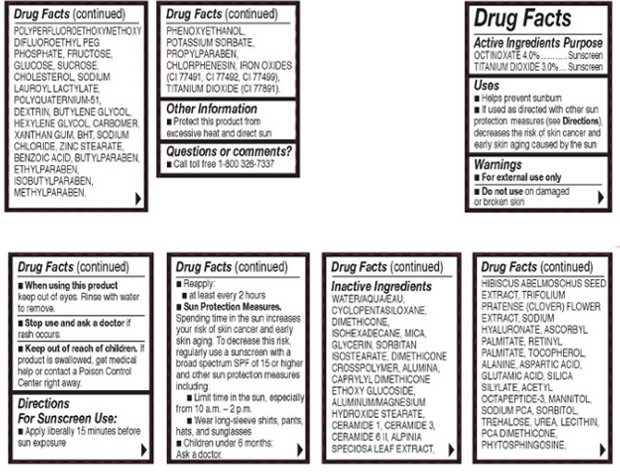
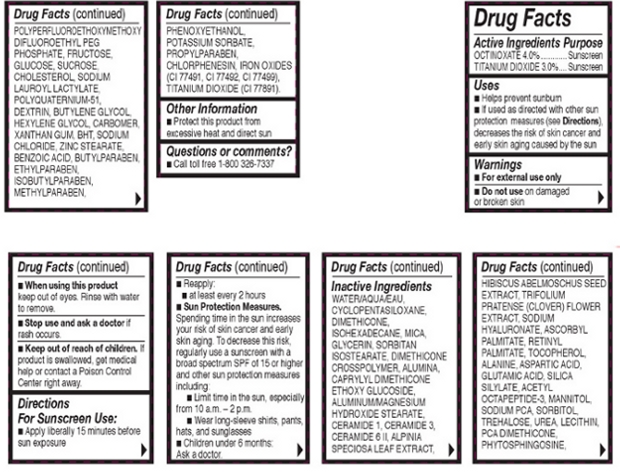
- OTC - ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Other Ingredients: Water/Aqua/Eau, Cyclopentasiloxane, Dimethicone, Isohexadecane, Mica, Glycerin, Sorbitan, Isostearate, Dimethicone Crosspolymer, Alumina, Caprylyl Dimethicone Ethoxy Glucoside, Aluminum/Magnesium Hydroxide Stearate, Ceramide 1, Ceramide 3, Ceramide 6 II, Alpinia Speciosa Leaf Extract, Hibiscus Abelmoschus Seed Extract, Trifolium Pratense (Clover) Flower Extract, Sodium Hyaluronate, Ascorbyl Palmitate, Retinyl palmitate, Tocopherol, Alanine, Aspartic Acide, Glumatic Acid, Silica Silyate, Acetyl Octatpeptide-3, Mannitol, Sodium PCA, Sorbitol, Trehalose, Urea, Lecithin, PCA Dimethicone, Phytosphingosine, Polyperfluoroethoxymethoxy Difluororethyl PEG Phostphate, Fructose, Glucose, Sucrose, Cholesterol, Sodium Lauroyl Lactylate, Polyquaternium-51, Dextrin, Butylene Glycol, Hexylene Glycol, Carbomer, Xanthan Gum, BHT, Sodium Choloride, Zinc Stearate, Benzoic Acid, Butylparaben, Ethylparaben, Isobutylparaben, Methylparaben, Phenoxyethanol, Pottasium Sorbate, Propylparaben, Chlorphenesin, Iron Oxides (CI 77481, CI 77482), Titanium Dioxide (CI 77891).
- DOSAGE & ADMINISTRATION
- OTC - KEEP OUT OF REACH OF CHILDREN
- OTC - PURPOSE
- OTC - WHEN USING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAMIDE LIFT AND FIRM MAKEUP BROAD SPECTRUM SUNSCREEN SPF 15 SPICE
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67938-0951 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.28 g in 32 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.96 g in 32 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISOHEXADECANE (UNII: 918X1OUF1E) MICA (UNII: V8A1AW0880) GLYCERIN (UNII: PDC6A3C0OX) CERAMIDE 3 (UNII: 4370DF050B) TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) ASCORBYL PALMITATE (UNII: QN83US2B0N) ALANINE (UNII: OF5P57N2ZX) ASPARTIC ACID (UNII: 30KYC7MIAI) GLUTAMIC ACID (UNII: 3KX376GY7L) MANNITOL (UNII: 3OWL53L36A) SORBITOL (UNII: 506T60A25R) TREHALOSE (UNII: B8WCK70T7I) UREA (UNII: 8W8T17847W) FRUCTOSE (UNII: 6YSS42VSEV) SUCROSE (UNII: C151H8M554) CHOLESTEROL (UNII: 97C5T2UQ7J) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HEXYLENE GLYCOL (UNII: KEH0A3F75J) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM CHLORIDE (UNII: 451W47IQ8X) ZINC STEARATE (UNII: H92E6QA4FV) BENZOIC ACID (UNII: 8SKN0B0MIM) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLPARABEN (UNII: Z8IX2SC1OH) CHLORPHENESIN (UNII: I670DAL4SZ) Product Characteristics Color BROWN (Spice) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67938-0951-1 1 in 1 BOX 1 NDC:67938-0951-2 32 g in 1 JAR 2 NDC:67938-0951-3 25000 g in 1 PAIL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 08/05/2010 Labeler - Elizabeth Arden, Inc (849222187)