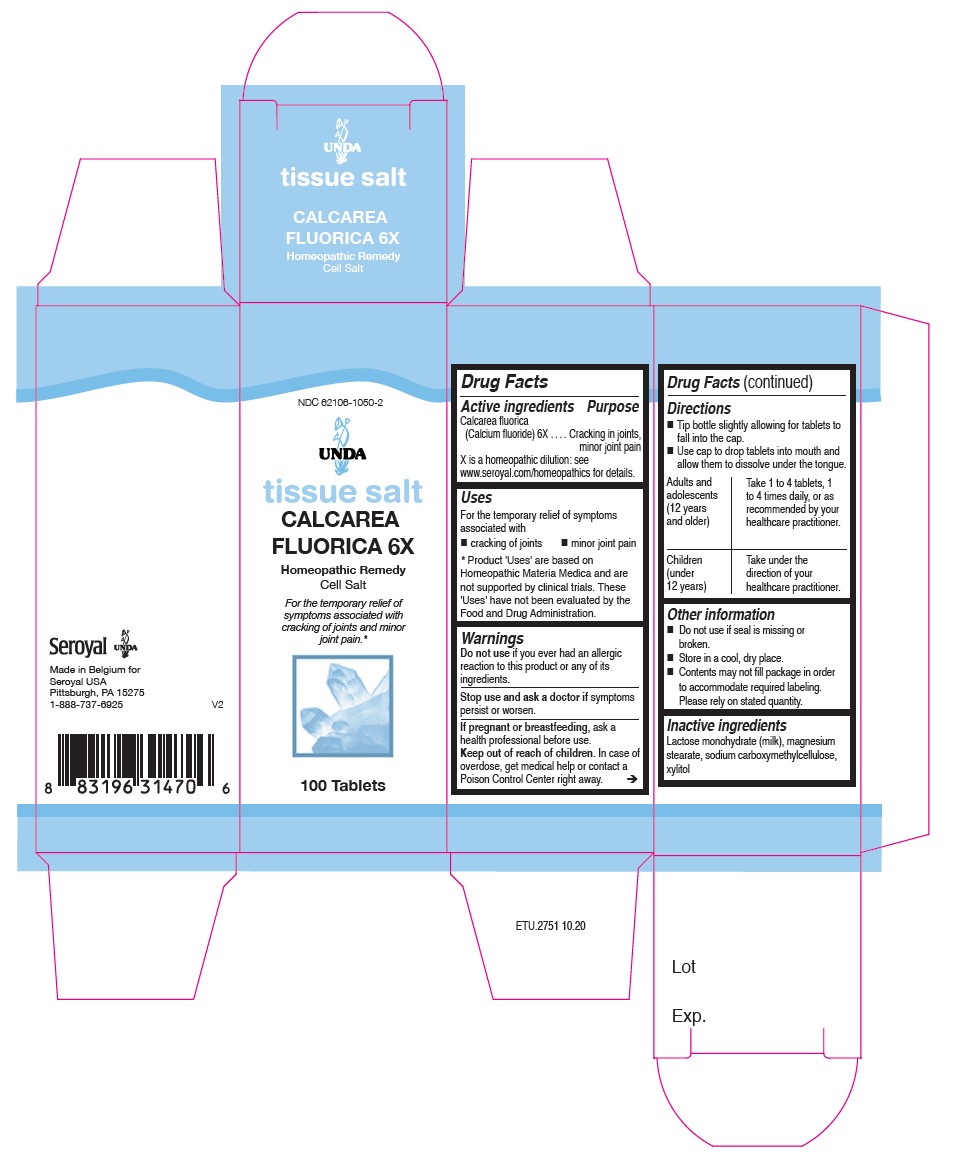
Label: CALCAREA FLUORICA 6X- calcium fluoride tablet
- NDC Code(s): 62106-1050-2
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 14, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Do not use if you ever had an allergic reaction to this product or any of its ingredients.
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a healthcare practitioner before use.
Keep out of reach of children.In case of overdose, get medical help or contact
a Poison Control Center right away. - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Directions
Tip bottle slightly allowing for tablets to fall into the cap.
Use cap to drop tablets into mouth and allow them to dissolve under the tongue.
Adults and adolescents (12 years and older)
Take 1 to 4 tablets, one to four times daily, or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated
with cracking of joints and minor joint pain.
Directions
Tip bottle slightly allowing for tablets to fall into the cap.
Use cap to drop tablets into mouth and allow them to dissolve under the tongue.
Adults and Adolescents (12 years and older): Take 1 to 4 tablets, one to
four times daily, or as recommended by your healthcare practitioner.Children (under 12 years): Take under the direction of your healthcare practitioner.
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALCAREA FLUORICA 6X
calcium fluoride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1050 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 6 [hp_X] Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) MAGNESIUM STEARATE (UNII: 70097M6I30) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color white Score no score Shape ROUND Size 7mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1050-2 1 in 1 BOX 10/02/2015 1 100 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/10/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN'UP 401010287 manufacture(62106-1050)